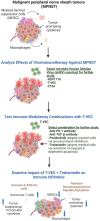
Myelomodulatory treatments augment the therapeutic benefit of oncolytic viroimmunotherapy in murine models of malignant peripheral nerve sheath tumors
- PMID: 39044819
- PMCID: PMC11263800
- DOI: 10.3389/fimmu.2024.1384623
Myelomodulatory treatments augment the therapeutic benefit of oncolytic viroimmunotherapy in murine models of malignant peripheral nerve sheath tumors
Abstract
Introduction: Malignant peripheral nerve sheath tumors (MPNST) pose a significant therapeutic challenge due to high recurrence rates after surgical resection and a largely ineffective response to traditional chemotherapy. An alternative treatment strategy is oncolytic viroimmunotherapy, which can elicit a durable and systemic antitumor immune response and is Food and Drug Administration (FDA)-approved for the treatment of melanoma. Unfortunately, only a subset of patients responds completely, underscoring the need to address barriers hindering viroimmunotherapy effectiveness.
Methods: Here we investigated the therapeutic utility of targeting key components of the MPNST immunosuppressive microenvironment to enhance viroimmunotherapy's antitumor efficacy in three murine models, one of which showed more immunogenic characteristics than the others.
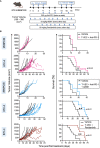
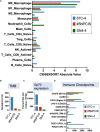
Results: Myelomodulatory therapy with pexidartinib, a small molecule inhibitor of CSF1R tyrosine kinase, and the oncolytic herpes simplex virus T-VEC exhibited the most significant increase in median survival time in the highly immunogenic model. Additionally, targeting myeloid cells with the myelomodulatory therapy trabectedin, a small molecule activator of caspase-8 dependent apoptosis, augmented the survival benefit of T-VEC in a less immunogenic MPNST model. However, tumor regressions or shrinkages were not observed. Depletion experiments confirmed that the enhanced survival benefit relied on a T cell response. Furthermore, flow cytometry analysis following combination viroimmunotherapy revealed decreased M2 macrophages and myeloid-derived suppressor cells and increased tumor-specific gp70+ CD8 T cells within the tumor microenvironment.
Discussion: In summary, our findings provide compelling evidence for the potential to leverage viroimmunotherapy with myeloid cell targeting against MPNST and warrant further investigation.
Keywords: T-VEC; immunotherapy; macrophage targeting; malignant peripheral nerve sheath tumors; oncolytic virotherapy; pexidartinib; trabectedin; tumor microenvironment.
Copyright © 2024 Paudel, Hutzen, Miller, Garfinkle, Chen, Wang, Glaspell, Currier, Ringwalt, Boon, Mardis, Cairo, Ratner, Dodd, Cassady and Cripe.
Conflict of interest statement
LB was employed by JJP Biologics. MSC has served as a consultant for Jazz Pharmaceuticals, Omeros Pharmaceuticals, Servier Pharmaceuticals, Abbvie and Novartis Pharmaceuticals; Speakers Bureau for Jazz Pharmaceuticals, Amgen, Inc., Sanofi and Sobi; Advisory Board for Astra Zeneca; and research funding from Celularity, Merck, Miltenyi Biotec, Servier, Omeros, Jazz and Janssen. KC holds intellectual property for oncolytic virus C134, which was licensed to Mustang Bio. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

