Castleman disease patients report mild COVID-19 symptoms and mount a humoral response to SARS-CoV-2 vaccination
- PMID: 39044861
- PMCID: PMC11265787
- DOI: 10.1016/j.bneo.2024.100002
Castleman disease patients report mild COVID-19 symptoms and mount a humoral response to SARS-CoV-2 vaccination
Abstract
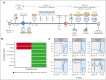
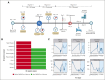
The coronavirus disease of 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has resulted in increased morbidity and mortality in patients with impaired immunity, hematologic malignancies, and immunosuppressive regimens. COVID-19 can cause a cytokine storm with some patients benefiting from blockade of the pro-inflammatory cytokine, interleukin 6 (IL6). As Castleman disease (CD) is an atypical lymphoproliferative disorder that can involve a cytokine storm and often requires immunosuppressive therapies, including IL6 inhibition, we sought to evaluate outcomes following COVID-19 and SARS-CoV-2 vaccination in CD patients. We administered a survey in April 2021 to characterize experiences with COVID-19 and SARS-CoV-2 vaccination among 300 CD patients enrolled in ACCELERATE, a natural history registry of CD patients. Among 128 respondents, the prevalence of SARS-CoV-2 infection (16/95, 17%), severe disease (1/16, 6%), vaccination rates (112/128, 88%), and vaccine adverse effects after dose one (62/112, 55%) were comparable to the general U.S. population. While there were two cases of CD flares occurring shortly after SARS-CoV-2 infection (N=1) and vaccination (N=1), over 100 patients in this study that were infected and/or vaccinated did not experience CD flares. The median anti-spike titer six months after the second dose among CD patients was comparable to individuals with other immune-related diseases and healthy populations. Data from this small cohort suggest that, despite being on immunosuppressive therapies, CD patients do not appear to be at increased risk of poor COVID-19 outcomes and can mount a humoral response to SARS-CoV-2 vaccination. This study was registered on clinicaltrials.gov (#NCT02817997).
Conflict of interest statement
Disclosure of Conflicts of Interest All other authors report no conflicts of interest.
Figures

References
-
- Fajgenbaum DC, Shilling D. Castleman disease pathogenesis. Hematol Oncol Clin North Am. 2018;32(1):11–21. - PubMed
-
- Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135(16):1353–1364. - PubMed
-
- van Rhee F, Greenway A, Stone K. Treatment of idiopathic Castleman disease. Hematol Oncol Clin North Am. 2018;32(1):89–106. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

