Neurological adverse events associated with oxaliplatin: A pharmacovigilance analysis based on FDA adverse event reporting system
- PMID: 39045045
- PMCID: PMC11263116
- DOI: 10.3389/fphar.2024.1431579
Neurological adverse events associated with oxaliplatin: A pharmacovigilance analysis based on FDA adverse event reporting system
Abstract
Objective: This study aimed to explore the neurological adverse events of oxaliplatin through the Food and Drug Administration Adverse Event Reporting System (FAERS) database and to provide reference for safe clinical drug use.
Methods: The adverse events report data of oxaliplatin from the first quarter of 2019 (1 January 2019) to the third quarter of 2023 (30 September 2023) were extracted from FAERS database, and the adverse events signal intensity was determined using the reporting odds ratio, proportional reporting ratio, information component, and empirical Bayes geometric mean methods. Time-to-onset and univariate logistic regression analysis were performed to describe the characteristics and risk factors of oxaliplatin-associated neurological adverse events.
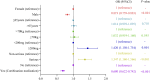
Results: A total of 4,471 cases of oxaliplatin-associated neurological adverse events were identified, with 318 neurological adverse events being documented, among which 87 adverse events satisfied the thresholds of four methodologies. The median time-to-onset of oxaliplatin-associated neurological adverse events was 2 days (interquartile range 0-36 days). Among the factors significantly influencing oxaliplatin-related neurological adverse events, male sex and combination medication decreased the risk of neurological adverse events, while higher cumulative dose increased the risk.
Conclusion: The real-world neurotoxicity spectrum of oxaliplatin and its characteristics and influencing factors were obtained through data mining of FAERS, providing valuable insights for healthcare professionals to effectively manage the risk of neurological adverse events associated with oxaliplatin in clinical practice.
Keywords: adverse event signals; date mining; neurological adverse events; oxaliplatin; the food and drug adverse event reporting system.
Copyright © 2024 Pan, Xiao, Ding, Shu, Zhang and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

