Therapeutic targeting of voltage-gated sodium channel NaV1.7 for cancer metastasis
- PMID: 39045054
- PMCID: PMC11263763
- DOI: 10.3389/fphar.2024.1416705
Therapeutic targeting of voltage-gated sodium channel NaV1.7 for cancer metastasis
Abstract
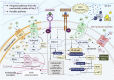
This review focuses on the expression and function of voltage-gated sodium channel subtype NaV1.7 in various cancers and explores its impact on the metastasis driving cell functions such as proliferation, migration, and invasiveness. An overview of its structural characteristics, drug binding sites, inhibitors and their likely mechanisms of action are presented. Despite the lack of clarity on the precise mechanism by which NaV1.7 contributes to cancer progression and metastasis; many studies have suggested a connection between NaV1.7 and proteins involved in multiple signaling pathways such as PKA and EGF/EGFR-ERK1/2. Moreover, the functional activity of NaV1.7 appears to elevate the expression levels of MACC1 and NHE-1, which are controlled by p38 MAPK activity, HGF/c-MET signaling and c-Jun activity. This cascade potentially enhances the secretion of extracellular matrix proteases, such as MMPs which play critical roles in cell migration and invasion activities. Furthermore, the NaV1.7 activity may indirectly upregulate Rho GTPases Rac activity, which is critical for cytoskeleton reorganization, cell adhesion, and actin polymerization. The relationship between NaV1.7 and cancer progression has prompted researchers to investigate the therapeutic potential of targeting NaV1.7 using inhibitors. The positive outcome of such studies resulted in the discovery of several inhibitors with the ability to reduce cancer cell migration, invasion, and tumor growth underscoring the significance of NaV1.7 as a promising pharmacological target for attenuating cancer cell proliferation and metastasis. The research findings summarized in this review suggest that the regulation of NaV1.7 expression and function by small molecules and/or by genetic engineering is a viable approach to discover novel therapeutics for the prevention and treatment of metastasis of cancers with elevated NaV1.7 expression.
Keywords: Nav1.7; cancer; cell invasion; cell migration; cell viability; metastasis; therapeutic targeting; voltage-gated sodium channel.
Copyright © 2024 Pukkanasut, Jaskula-Sztul, Gomora and Velu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- American Cancer Society (2023). Cancer fact & figure. Atlanta: American Cancer Society. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-... (Accessed February 8, 2024).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

