Inhibiting retinoic acid signaling in dendritic cells suppresses respiratory syncytial virus infection through enhanced antiviral immunity
- PMID: 39045100
- PMCID: PMC11263793
- DOI: 10.1016/j.isci.2024.110103
Inhibiting retinoic acid signaling in dendritic cells suppresses respiratory syncytial virus infection through enhanced antiviral immunity
Abstract
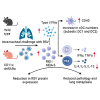
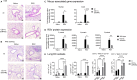
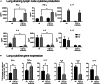
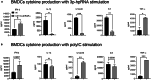
Retinoic acid (RA), controls the immunoregulatory functions of many immune cells, including dendritic cells (DCs), and is important for mucosal immunity. In DCs, RA regulates the expression of pattern recognition receptors and stimulates interferon production. Here, we investigated the role of RA in DCs in mounting immunity to respiratory syncytial virus (RSV). To abolish RA signaling in DCs, we used mice expressing a dominant negative form of retinoic acid receptor-α (RARα) under the CD11c promoter (CD11c-dnRARα). Paradoxically, upon RSV challenge, these animals had lower viral burden, reduced pathology, and greater Th1 polarized immunity than wild-type (WT) mice. Moreover, CD11c-dnRARα DCs infected with RSV showed enhancement in innate and adaptive immunity genes, while genes associated with viral replication were downregulated. These findings suggest that the absence of RA signaling in DCs enhances innate immunity against RSV infection leading to decreased viral load and reduced pathogenicity.
Keywords: Immunology; Virology; cell biology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection.Viruses. 2019 Jan 15;11(1):62. doi: 10.3390/v11010062. Viruses. 2019. PMID: 30650519 Free PMC article.
-
Immunobiotic Lactobacillus rhamnosus improves resistance of infant mice against respiratory syncytial virus infection.Int Immunopharmacol. 2013 Oct;17(2):373-82. doi: 10.1016/j.intimp.2013.06.024. Epub 2013 Jul 7. Int Immunopharmacol. 2013. PMID: 23838113
-
Effects of respiratory syncytial virus infection on dendritic cells and cysteinyl leukotrienes in lung tissues of a murine model of asthma.Allergol Int. 2007 Jun;56(2):165-9. doi: 10.2332/allergolint.O-06-476. Epub 2007 May 1. Allergol Int. 2007. PMID: 17460444
-
Induction and Subversion of Human Protective Immunity: Contrasting Influenza and Respiratory Syncytial Virus.Front Immunol. 2018 Mar 2;9:323. doi: 10.3389/fimmu.2018.00323. eCollection 2018. Front Immunol. 2018. PMID: 29552008 Free PMC article. Review.
-
Contribution of Dendritic Cells in Protective Immunity against Respiratory Syncytial Virus Infection.Viruses. 2020 Jan 15;12(1):102. doi: 10.3390/v12010102. Viruses. 2020. PMID: 31952261 Free PMC article. Review.
Cited by
-
Short-Chain Fatty Acids: Promising Therapeutic Targets for Respiratory Syncytial Virus Infection.Clin Rev Allergy Immunol. 2025 Jan 28;68(1):8. doi: 10.1007/s12016-024-09018-x. Clin Rev Allergy Immunol. 2025. PMID: 39873814 Review.
References
-
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O'Brien K.L., Roca A., Wright P.F., Bruce N., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Zhang L., Peeples M.E., Boucher R.C., Collins P.L., Pickles R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002;76:5654–5666. doi: 10.1128/jvi.76.11.5654-5666.2002. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

