Treatment with bulevirtide in HIV-infected patients with chronic hepatitis D: ANRS HD EP01 BuleDelta and compassionate cohort
- PMID: 39045338
- PMCID: PMC11264178
- DOI: 10.1016/j.jhepr.2024.101057
Treatment with bulevirtide in HIV-infected patients with chronic hepatitis D: ANRS HD EP01 BuleDelta and compassionate cohort
Abstract
Background & aims: In France, bulevirtide (BLV) became available in September 2019 through an early access program to treat patients with HDV. The aim of this analysis was to evaluate the efficacy and safety of BLV in patients with HIV and HDV coinfection.
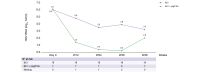
Methods: Patients received BLV 2 mg ± pegylated interferon-α (pegIFNα) according to the physician's decision. The primary endpoint (per-protocol analysis) was the virological response rate at Week 48, defined as the proportion of patients with undetectable serum HDV RNA or a HDV RNA decline >2 log10 IU/ml from baseline.
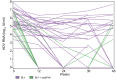
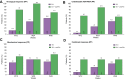
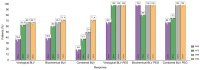
Results: The characteristics of the 38 patients were as follows: 28 male, mean age 47.7 years, and mean baseline HDV RNA viral load 5.7 ± 1.2 log10 IU/ml. Median HIV viral load and mean CD4 count were 32 (30-65) copies/ml and 566 ± 307/mm3, respectively. Eight patients stopped treatment before Week 48. At Week 48, 10 of 19 patients (52.6%) in the 2 mg BLV group and five of seven patients (71.4%) in the 2 mg BLV + pegIFNɑ group had reached virological response (no HDV RNA available in four patients). At Week 48, seven of 19 patients in the 2 mg BLV group and three of six patients in the 2 mg BLV + pegIFNɑ group had a combined response (virological response and normal alanine aminotransferase level).
Conclusions: Adults living with HIV coinfected with HDV can be treated by BLV with a virological response in more than 50% of patients. The combination of BLV and pegIFNɑ showed a strong virological response.
Impact and implications: Bulevirtide is the only EMA-approved drug for HDV treatment, and we showed that it can be used in adults living with HIV, with an overall good tolerability. Bulevirtide induces a virological response in more than 50% of patients, suggesting that bulevirtide should be considered as a first-line therapy in this specific population. Bulevirtide in combination with pegIFNα could be used in patients without pegIFNα contraindication. No specific drug-drug interaction is reported. Bulevirtide is the only EMA-approved drug for HDV treatment, and we showed that it can be used in adults living with HIV, with an overall good tolerability. Bulevirtide induces a virological response in more than 50% of patients, suggesting that bulevirtide should be considered as a first-line therapy in this specific population. Bulevirtide in combination with pegIFNα could be used in patients without pegIFNα contraindication. No specific drug-drug interaction is reported. Bulevirtide is the only EMA-approved drug for HDV treatment, and we showed that it can be used in adults living with HIV, with an overall good tolerability. Bulevirtide induces a virological response in more than 50% of patients, suggesting that bulevirtide should be considered as a first-line therapy in this specific population. Bulevirtide in combination with pegIFNα could be used in patients without pegIFNα contraindication. No specific drug-drug interaction is reported.
Keywords: Cirrhosis; Entry inhibitors; HBV; HBV DNA; HDV; HDV RNA; HIV; HIV RNA; Hepatitis D; Pegylated interferon.
© 2024 The Author(s).
Figures

References
-
- Asselah T., Rizzetto M. Hepatitis D virus infection. N Engl J Med. 2023;389:58–70. - PubMed
-
- Piroth L., Pol S., Lacombe K., et al. Management and treatment of chronic hepatitis B virus infection in HIV positive and negative patients: the EPIB 2008 study. J Hepatol. 2010;53:1006–1012. - PubMed
-
- Alfaiate D., Clément S., Gomes D., et al. Chronic hepatitis D and hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. J Hepatol. 2020;73:533–539. - PubMed
-
- Wedemeyer H., Yurdaydin C., Hardtke S., et al. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19:275–286. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

