Molecular pathophysiology of secondary lymphedema
- PMID: 39045461
- PMCID: PMC11264244
- DOI: 10.3389/fcell.2024.1363811
Molecular pathophysiology of secondary lymphedema
Abstract
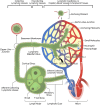
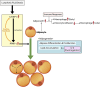
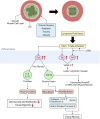
Lymphedema occurs as a result of lymphatic vessel damage or obstruction, leading to the lymphatic fluid stasis, which triggers inflammation, tissue fibrosis, and adipose tissue deposition with adipocyte hypertrophy. The treatment of lymphedema is divided into conservative and surgical approaches. Among surgical treatments, methods like lymphaticovenular anastomosis and vascularized lymph node transfer are gaining attention as they focus on restoring lymphatic flow, constituting a physiologic treatment approach. Lymphatic endothelial cells form the structure of lymphatic vessels. These cells possess button-like junctions that facilitate the influx of fluid and leukocytes. Approximately 10% of interstitial fluid is connected to venous return through lymphatic capillaries. Damage to lymphatic vessels leads to lymphatic fluid stasis, resulting in the clinical condition of lymphedema through three mechanisms: Inflammation involving CD4+ T cells as the principal contributing factor, along with the effects of immune cells on the VEGF-C/VEGFR axis, consequently resulting in abnormal lymphangiogenesis; adipocyte hypertrophy and adipose tissue deposition regulated by the interaction of CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor-γ; and tissue fibrosis initiated by the overactivity of Th2 cells, leading to the secretion of profibrotic cytokines such as IL-4, IL-13, and the growth factor TGF-β1. Surgical treatments aimed at reconstructing the lymphatic system help facilitate lymphatic fluid drainage, but their effectiveness in treating already damaged lymphatic vessels is limited. Therefore, reviewing the pathophysiology and molecular mechanisms of lymphedema is crucial to complement surgical treatments and explore novel therapeutic approaches.
Keywords: adipose tissue; fibrosis; inflammation; lymphatic system; lymphedema; molecular biology; physiopathology.
Copyright © 2024 Lee and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aschen S. Z., Farias-Eisner G., Cuzzone D. A., Albano N. J., Ghanta S., Weitman E. S., et al. (2014). Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow. Plast. Reconstr. Surg. 133 (2), 301–310. 10.1097/01.prs.0000436840.69752.7e - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

