Immune-related adverse events in patients treated with immunotherapy for locally advanced or metastatic NSCLC in real-world settings: a systematic review and meta-analysis
- PMID: 39045561
- PMCID: PMC11263096
- DOI: 10.3389/fonc.2024.1415470
Immune-related adverse events in patients treated with immunotherapy for locally advanced or metastatic NSCLC in real-world settings: a systematic review and meta-analysis
Abstract
Introduction: Randomized clinical trials (RCTs) represent the mainstay for the approval of new treatments. However, stringent inclusion criteria often cause them to depart from the daily clinical practice. Real-world (RW) evidence have a complementing role, filling the gap between the efficacy of a treatment and its effectiveness. Immune checkpoint inhibitors (ICIs) have changed the treatment scenario for non-small cell lung cancer (NSCLC); immune-related adverse events (irAEs) could become life-threatening events, when not timely managed. We performed a systematic review and meta-analysis on the RW impact of irAEs through the years.
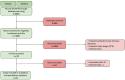
Methods: The systematic review focused on irAEs occurred in locally advanced or metastatic NSCLC patients, treated with ICIs in a RW setting. We queried two electronic databases (Embase and Medline) from 1996 to August 2022. We then conducted a meta-analysis dividing the results in two cohorts (2015-2018 and 2019-2021). We described the prevalence of patients with irAEs of any or severe grade (G). Estimates were expressed as proportions up to the second decimal point (effect size, ES). IrAEs of interest were those involving the skin, the liver, the endocrine system or the gastro-intestinal system.
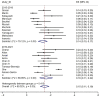
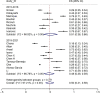
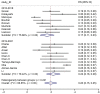
Results: Overall, 21 RW studies on 5,439 patients were included in the quantitative and qualitative synthesis. The prevalence of G≥3 irAEs was slightly lower in the 2015-2018 subgroup, while the prevalence of irAEs of any grade was similar for both periods. Overall, we observed a higher ES for gastrointestinal, hepatic and lung irAEs, while a lower ES was reported for skin or endocrine irAEs. Endocrine irAEs were reported in 10 out of 21 studies, with a slight increase in the most recent studies, while cutaneous toxicities were mostly reported in two studies lead within the first time-period. Pulmonary, gastrointestinal, and hepatic toxicities, showed a more heterogeneous distribution of ES over time.
Discussion: Our findings showed that the frequency of irAEs remained stable across the two calendar periods examined in our meta-analysis. This finding suggests that RW data might not be able to identify a potential learning curve in detection and management of irAEs.
Keywords: immune-related adverse events; immunotherapy; meta-analysis; non-small cell lung cancer; real world evidence.
Copyright © 2024 Pasello, Pavan, De Nuzzo, Frega, Ferro, Dal Maso, Bonanno, Guarneri and Girardi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7 - DOI - PubMed
-
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

