Lack of Bmal1 leads to changes in rhythmicity and impairs motivation towards natural stimuli
- PMID: 39045857
- PMCID: PMC11267724
- DOI: 10.1098/rsob.240051
Lack of Bmal1 leads to changes in rhythmicity and impairs motivation towards natural stimuli
Abstract
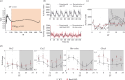
Maintaining proper circadian rhythms is essential for coordinating biological functions in mammals. This study investigates the effects of daily arrhythmicity using Bmal1-knockout (KO) mice as a model, aiming to understand behavioural and motivational implications. By employing a new mathematical analysis based on entropy divergence, we identified disrupted intricate activity patterns in mice derived by the complete absence of BMAL1 and quantified the difference regarding the activity oscillation's complexity. Changes in locomotor activity coincided with disturbances in circadian gene expression patterns. Additionally, we found a dysregulated gene expression profile particularly in brain nuclei like the ventral striatum, impacting genes related to reward and motivation. Further investigation revealed that arrhythmic mice exhibited heightened motivation for food and water rewards, indicating a link between circadian disruptions and the reward system. This research sheds light on how circadian clock alterations impact the gene expression regulating the reward system and how this, in turn, can lead to altered seeking behaviour and motivation for natural rewards. In summary, the present study contributes to our understanding of how reward processing is under the regulation of circadian clock machinery.
Keywords: Bmal1; circadian disruption; daily rhythms; natural reinforcer; reward system.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

