Strategies for cessation of caffeine administration in preterm infants
- PMID: 39045901
- PMCID: PMC11267609
- DOI: 10.1002/14651858.CD015802.pub2
Strategies for cessation of caffeine administration in preterm infants
Abstract
Background: Apnea and intermittent hypoxemia (IH) are common developmental disorders in infants born earlier than 37 weeks' gestation. Caffeine administration has been shown to lower the incidence of these disorders in preterm infants. Cessation of caffeine treatment is based on different post-menstrual ages (PMA) and resolution of symptoms. There is uncertainty about the best timing for caffeine discontinuation.
Objectives: To evaluate the effects of early versus late discontinuation of caffeine administration in preterm infants.
Search methods: We searched CENTRAL, PubMed, Embase, and three trial registries in August 2023; we applied no date limits. We checked the references of included studies and related systematic reviews.
Selection criteria: We included randomized controlled trials (RCTs) in preterm infants born earlier than 37 weeks' gestation, up to a PMA of 44 weeks and 0 days, who received caffeine for any indication for at least seven days. We compared three different strategies for caffeine cessation: 1. at different PMAs, 2. before or after five days without symptoms, and 3. at a predetermined PMA versus at the resolution of symptoms.
Data collection and analysis: We used standard Cochrane methods. Primary outcomes were: restarting caffeine therapy, intubation within one week of treatment discontinuation, and the need for non-invasive respiratory support within one week of treatment discontinuation. Secondary outcomes were: number of episodes of apnea in the seven days after treatment discontinuation, number of infants with at least one episode of apnea in the seven days after treatment discontinuation, number of episodes of intermittent hypoxemia (IH) within seven days of treatment discontinuation, number of infants with at least one episode of IH in the seven days after of treatment discontinuation, all-cause mortality prior to hospital discharge, major neurodevelopmental disability, number of days of respiratory support after treatment discontinuation, duration of hospital stay, and cost of neonatal care. We used GRADE to assess the certainty of evidence for each outcome.
Main results: We included three RCTs (392 preterm infants). Discontinuation of caffeine at PMA less than 35 weeks' gestation versus PMA equal to or longer than 35 weeks' gestation This comparison included one single completed RCT with 98 premature infants with a gestational age between 25 + 0 and 32 + 0 weeks at birth. All infants had discontinued caffeine treatment for five days at randomization. The infants received either an oral loading dose of caffeine citrate (20 mg/kg) at randomization followed by oral maintenance dosage (6 mg/kg/day) until 40 weeks PMA, or usual care (controls), during which caffeine was stopped before 37 weeks PMA. Early cessation of caffeine administration in preterm infants at PMA less than 35 weeks' gestation may result in an increase in the number of IH episodes in the seven days after discontinuation of treatment, compared to prolonged caffeine treatment beyond 35 weeks' gestation (mean difference [MD] 4.80, 95% confidence interval [CI] 2.21 to 7.39; 1 RCT, 98 infants; low-certainty evidence). Early cessation may result in little to no difference in all-cause mortality prior to hospital discharge compared to late discontinuation after 35 weeks PMA (risk ratio [RR] not estimable; 98 infants; low-certainty evidence). No data were available for the following outcomes: restarting caffeine therapy, intubation within one week of treatment discontinuation, need for non-invasive respiratory support within one week of treatment discontinuation, number of episodes of apnea, number of infants with at least one episode of apnea in the seven days after discontinuation of treatment, or number of infants with at least one episode of IH in the seven days after discontinuation of treatment. Discontinuation based on PMA versus resolution of symptoms This comparison included two RCTs with a total of 294 preterm infants. Discontinuing caffeine at the resolution of symptoms compared to discontinuing treatment at a predetermined PMA may result in little to no difference in all-cause mortality prior to hospital discharge (RR 1.00, 95% CI 0.14 to 7.03; 2 studies, 294 participants; low-certainty evidence), or in the number of infants with at least one episode of apnea within the seven days after discontinuing treatment (RR 0.60, 95% CI 0.31 to 1.18; 2 studies; 294 infants; low-certainty evidence). Discontinuing caffeine based on the resolution of symptoms probably results in more infants with IH in the seven days after discontinuation of treatment (RR 0.38, 95% CI 0.20 to 0.75; 1 study; 174 participants; moderate-certainty evidence). No data were available for the following outcomes: restarting caffeine therapy, intubation within one week of treatment discontinuation, need for non-invasive respiratory support within one week of treatment discontinuation, or number of episodes of IH in the seven days after treatment discontinuation. Adverse effects In the Rhein 2014 study, five of the infants randomized to caffeine had the caffeine treatment discontinued at the discretion of the clinical team, because of tachycardia. The Pradhap 2023 study reported adverse events, including recurrence of apnea of prematurity (15% in the short and 13% in the regular course caffeine therapy group), varying severities of bronchopulmonary dysplasia, hyperglycemia, extrauterine growth restriction, retinopathy of prematurity requiring laser treatment, feeding intolerance, osteopenia, and tachycardia, with no significant differences between the groups. The Prakash 2021 study reported that adverse effects of caffeine therapy for apnea of prematurity included tachycardia, feeding intolerance, and potential neurodevelopmental impacts, though most were mild and transient. We identified three ongoing studies.
Authors' conclusions: There may be little or no difference in the incidence of all-cause mortality and apnea in infants who were randomized to later discontinuation of caffeine treatment. However, the number of infants with at least one episode of IH was probably reduced with later cessation. No data were found to evaluate the benefits and harms of later caffeine discontinuation for: restarting caffeine therapy, intubation within one week of treatment discontinuation, or need for non-invasive respiratory support within one week of treatment discontinuation. Further studies are needed to evaluate the short-term and long-term effects of different caffeine cessation strategies in premature infants.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
EB, MG, RC, and SU have no interests to declare.
MB is an Associate Editor for the Cochrane Neonatal Group. However, he had no involvement in the editorial processing of this protocol.
Figures
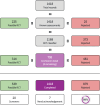
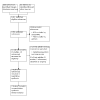






Update of
- doi: 10.1002/14651858.CD015802
Similar articles
-
Doxapram for the prevention and treatment of apnea in preterm infants.Cochrane Database Syst Rev. 2023 Oct 25;10(10):CD014145. doi: 10.1002/14651858.CD014145.pub2. Cochrane Database Syst Rev. 2023. PMID: 37877431 Free PMC article.
-
Continuous positive airway pressure versus methylxanthine for apnoea in preterm infants.Cochrane Database Syst Rev. 2025 Jul 22;7(7):CD001072. doi: 10.1002/14651858.CD001072.pub2. Cochrane Database Syst Rev. 2025. PMID: 40693523 Review.
-
Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome.Cochrane Database Syst Rev. 2024 Jan 25;1(1):CD008309. doi: 10.1002/14651858.CD008309.pub3. Cochrane Database Syst Rev. 2024. PMID: 38270182 Free PMC article.
-
Methylxanthine for the prevention and treatment of apnea in preterm infants.Cochrane Database Syst Rev. 2023 Oct 31;10(10):CD013830. doi: 10.1002/14651858.CD013830.pub2. Cochrane Database Syst Rev. 2023. PMID: 37905735 Free PMC article.
-
Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2021 Nov 11;11(11):CD001145. doi: 10.1002/14651858.CD001145.pub5. Cochrane Database Syst Rev. 2021. PMID: 34758507 Free PMC article.
Cited by
-
Extended Caffeine for Apnea in Moderately Preterm Infants: The MoCHA Randomized Clinical Trial.JAMA. 2025 Jun 24;333(24):2154-2163. doi: 10.1001/jama.2025.5791. JAMA. 2025. PMID: 40294395 Clinical Trial.
-
Caffeine: The Story beyond Oxygen-Induced Lung and Brain Injury in Neonatal Animal Models-A Narrative Review.Antioxidants (Basel). 2024 Sep 3;13(9):1076. doi: 10.3390/antiox13091076. Antioxidants (Basel). 2024. PMID: 39334735 Free PMC article. Review.
-
Caffeine and preterm infants: multiorgan effects and therapeutic creep: scope to optimise dose and timing.Pediatr Res. 2025 Jun 20. doi: 10.1038/s41390-025-04066-1. Online ahead of print. Pediatr Res. 2025. PMID: 40542101 Review.
References
References to studies included in this review
Pradhap 2023 {published data only}
-
- CTRI/2021/09/036141. How long is caffeine duration enough for preterm infants? https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/09/036141 (registered 02 September 2021).
-
- Pradhap K, Solaiappan M, Ramya S, Muthukumaran N. Effect of short course caffeine on recurrenceof apnea of prematurity in preterm infants lesst han 32 weeks: a randomized controlled trial. Journal of Neonatology 2023;0(0):1-7. [DOI: 10.1177/09732179231198302] - DOI
Prakash 2021 {published data only}
-
- CTRI/2016/12/007559. A clinical trial to evaluate the duration of caffeine therapy for apnea of prematurity. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2016/12/007559 (registered 9 December 2016).
Rhein 2014 {published data only}
-
- NCT01875159. Effects of caffeine on intermittent hypoxia in infants born preterm. https://clinicaltrials.gov/show/NCT01875159 (first submitted 06 March 2013).
References to studies excluded from this review
ACTRN12622001344785 {published data only}
-
- ACTRN12622001344785. Investigating the effect of caffeine to improve neurodevelopmental outcomes in babies born late preterm: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=384799 (first submitted 11 October 2022).
References to ongoing studies
NCT03321734 {published data only}
-
- NCT03321734. Intermittent hypoxia and caffeine in infants born preterm. https://classic.clinicaltrials.gov/ct2/show/NCT03321734 (first submitted 23 October 2017).
NCT03340727 {published data only}
-
- NCT03340727. Moderately preterm infants with caffeine at home for apnea (MoCHA) trial. https://clinicaltrials.gov/study/NCT03340727 (first submitted 08 Novmeber 2017).
NCT04868565 {published data only}
-
- NCT04868565. Target weaning oxygen to determine cafffeine duration for AOP. https://clinicaltrials.gov/study/NCT04868565?tab=table (first submitted 27 April 2021).
Additional references
Abdel‐Hady 2015
Aranda 1977
Aranda 1979
Arroyo 2021
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development-II. San Antonio (TX): Psychological Corporation, 1993.
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio (TX): Harcourt Assessment, 2006.
Blanchard 1992
Borenstein 2013
Brady 2019
Brattström 2019
Bruschettini 2023
Bui 2017
Butler 2014
-
- Butler TJ, Firestone KS, Grow JL, Kantak AD. Standardizing documentation and the clinical approach to apnea of prematurity reduces length of stay, improves staff satisfaction, and decreases hospital cost. Joint Commission Journal on Quality and Patient Safety 2014;40(6):263-9. [DOI: 10.1016/s1553-7250(14)40035-7] [PMID: ] - DOI - PubMed
Charles 2008
-
- Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Therapeutic Drug Monitoring 2008;30(6):709-16. [DOI: 10.1097/FTD.0b013e3181898b6f] [PMID: ] - DOI - PubMed
Chavez 2022
Chung 2022
-
- Chung J, Tran Lopez K, Amendolia B, Bhat V, Nakhla T, Slater-Myer L, et al. Stopping caffeine in premature neonates: how long does it take for the level of caffeine to fall below the therapeutic range? Journal of Maternal-Fetal & Neonatal Medicine 2022;35(3):551-5. [DOI: 10.1080/14767058.2020.1729117] - DOI - PubMed
Covidence [Computer program]
-
- Covidence. Version accessed 13 February 2023. Melbourne, Australia: Veritas Health Innovation, 2023. Available at https://www.covidence.org.
Crossley 2012
Darlow 2016
Darnall 1997
Deeks 2023
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from https://training.cochrane.org/handbook.
Dobson 2013
-
- Dobson NR, Hunt CE. Pharmacology review: caffeine use in neonates: indications, pharmacokinetics. NeoReviews 2013;14(11):e540–50. [DOI: 10.1542/neo.14-11-e540] - DOI
Doyle 2016
Doyle 2021
-
- Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL, Soll R. Early (< 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD001146. [DOI: 10.1002/14651858.CD001146.pub6] - DOI - PMC - PubMed
Dukhovny 2011
Egger 1997
Eichenwald 2016
EMA 2009
-
- Chiesi Farmaceutici SpA. European Public Assessment Report Nymusa/Peyona. Doc.Ref.: EMEA/CHMP/207328/2009. https://www.ema.europa.eu/en/medicines/human/EPAR/peyona-previously-nymu... (accessed 1 February 2023).
EMA 2020
-
- EMA. European Public Assessment Report Gencebok. EMA/353540/2020 27/08/2020. https://www.ema.europa.eu/en/medicines/human/EPAR/gencebok (accessed 1 February 2023).
Endesfelder 2020
EPOC 2017
-
- Cochrane Effective Practice and Organisation of Care Review Group. Data collection checklist. Available at https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/uploa... (accessed 12 July 2023).
Fewell 2007
Gentle 2018
Gillot 1990
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 15 February 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2023. Available at https://www.gradepro.org/.
Griffiths 1954
-
- Griffiths R. Abilities of Babies: A Study of Mental Measurement. London (UK): University of London Press, 1954.
Haddad 2015
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from https://training.cochrane.org/handbook/archive/v5.2.
Higgins 2023
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated August 2023). Cochrane, 2023. Available from https://training.cochrane.org/handbook.
Hunt 2011
-
- Hunt CE, Corwin MJ, Weese-Mayer DE, Ward SL, Ramanathan R, Lister G, et al, Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. Journal of Pediatrics 2011;159(3):377-83.e1. [DOI: 10.1016/j.jpeds.2011.02.011] [PMID: ] - DOI - PMC - PubMed
Isayama 2016
Jacobs 2013
Jefferies 2014
Jensen 2015
-
- Jensen EA, Foglia EE, Schmidt B. Evidence-based pharmacologic therapies for prevention of bronchopulmonary dysplasia: application of the grading of recommendations assessment, development, and evaluation methodology. Clinics in Perinatology 2015;42(4):755-79. [DOI: 10.1016/j.clp.2015.08.005] [PMID: ] - DOI - PubMed
Ji 2020
Jobe 2001
Kumar 2019
Kyriacou 2016
Lee 1997
Lemyre 2017
-
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub3] [PMID: ] - DOI - PMC - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1136/bmj.b2700] [PMID: ] - DOI - PMC - PubMed
Ma 2020
Marques 2023
Marshall 2018
Martin 2011
Martin 2022
-
- Martin R. Management of apnea of prematurity. Available at https://www.uptodate.com/contents/management-of-apnea-of-prematurity (accessed 12 July 2023).
McGowan 2016a
-
- McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&E). Ottawa (ON): CADTH, 2016. - PubMed
McGowan 2016b
Moresco 2023
-
- Moresco L, Sjögren A, Marques K, Soll R, Bruschettini M. Caffeine versus other methylxanthines for the prevention and treatment of apnea in preterm infants. Cochrane Database of Systematic Reviews 2023, Issue 10. Art. No: CD015462. [DOI: 10.1002/14651858.CD015462.pub2] [PMCID: PMC10548499] [PMID: ] - DOI - PMC - PubMed
Nagatomo 2016
NDA 20‐793/S‐001
-
- NDA 20-793/S-001 (2000). CAFCIT (caffeine citrate) Injection CAFCIT® (caffeine citrate) oral solution. accessdata.fda.gov/drugsatfda_docs/label/2000/20793s1lbl.pdf (accessed 1 February 2023).
NIH 1979
-
- National Institutes of Health (NIH). Report of Workshop on Bronchopulmonary Dysplasia; NIH Publication No. 80-1660. Washington (DC): National Institutes of Health, 1979.
Noel‐Storr 2020
-
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] - DOI - PubMed
Noel‐Storr 2021
-
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] - DOI - PubMed
Pierro 2017
Poggi 2014
Principi 2018
Ramanathan 2001
-
- Ramanathan R, Corwin MJ, Hunt CE, Lister G, Tinsley LR, Baird T, et al, Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. Cardiorespiratory events recorded on home monitors: comparison of healthy infants with those at increased risk for SIDS. JAMA 2001;285(17):2199-207. [DOI: 10.1001/jama.285.17.2199] - DOI - PubMed
Resch 2011
RevMan 2024 [Computer program]
-
- Review Manager (RevMan). Version 7.12. The Cochrane Collaboration, 2024. Available at https://revman.cochrane.org.
Rhein 2014
-
- Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF, et al, Caffeine Pilot Study Group. Caffeine pilot study group. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatrics 2014;168(3):250-7. [DOI: 10.1001/jamapediatrics.2013.4371] [PMID: ] - DOI - PubMed
Robertson 2009
Rostas 2019
Saroha 2020
Sauer‐Zavala 2019
-
- Sauer-Zavala S, Wilner J, Cassiello-Robbins C, Saraff P, Pagan D. Isolating the effect of opposite action in borderline personality disorder: a laboratory-based alternating treatment design. Behaviour Research and Therapy 2019;117:79-86. [DOI: 10.1016/j.brat.2018.10.006] [PMID: ] - DOI - PMC - PubMed
Schmidt 2006
Schmidt 2007
Schmidt 2012
-
- Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, et al, Caffeine for Apnea of Prematurity Trial Investigators. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012;307(3):275-82. [DOI: 10.1001/jama.2011.2024] [PMID: ] - DOI - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from https://gdt.gradepro.org/app/handbook/handbook.html.
Shrestha 2017
Stevenson 2007
Stoll 2015
-
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314(10):1039–51. [DOI: 10.1001/jama.2015.10244] [PMID: ] - DOI - PMC - PubMed
Tabacaru 2017
Thomas 2021
-
- Thomas J, McDonald S, Noel-Storr AH, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2021;133:140-51. [DOI: 10.1016/j.jclinepi.2020.11.003] [PMID: ] - DOI - PMC - PubMed
Thébaud 2019
Townsi 2018
Walsh 2004
-
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

