Regulation of Absorption and Emission in a Protein/Fluorophore Complex
- PMID: 39046136
- PMCID: PMC11334107
- DOI: 10.1021/acschembio.4c00125
Regulation of Absorption and Emission in a Protein/Fluorophore Complex
Abstract
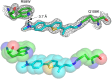
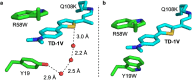
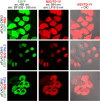
Human cellular retinol binding protein II (hCRBPII) was used as a protein engineering platform to rationally regulate absorptive and emissive properties of a covalently bound fluorogenic dye. We demonstrate the binding of a thio-dapoxyl analog via formation of a protonated imine between an active site lysine residue and the chromophore's aldehyde. Rational manipulation of the electrostatics of the binding pocket results in a 204 nm shift in absorption and a 131 nm shift in emission. The protein is readily expressed in mammalian systems and binds with exogenously delivered fluorophore as demonstrated by live-cell imaging experiments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Hong G.; Antaris A. L.; Dai H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010.10.1038/s41551-016-0010. - DOI
-
- Adams S. R.; Campbell R. E.; Gross L. A.; Martin B. R.; Walkup G. K.; Yao Y.; Llopis J.; Tsien R. Y. New Biarsenical Ligands and Tetracysteine Motifs for Protein Labeling in Vitro and in Vivo: Synthesis and Biological Applications. J. Am. Chem. Soc. 2002, 124, 6063–6076. 10.1021/ja017687n. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

