Photothermal Temperature-Modulated Cancer Metastasis Harnessed Using Proteinase-Triggered Assembly of Near-Infrared II Photoacoustic/Photothermal Nanotheranostics
- PMID: 39046148
- PMCID: PMC11310908
- DOI: 10.1021/acsami.4c07173
Photothermal Temperature-Modulated Cancer Metastasis Harnessed Using Proteinase-Triggered Assembly of Near-Infrared II Photoacoustic/Photothermal Nanotheranostics
Abstract
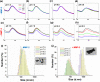
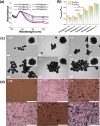
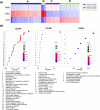
Here we demonstrate that cancer metastasis could be modulated by the judicious tuning of physical parameters such as photothermal temperature in nanoparticle-mediated photothermal therapy (PTT). This is supported by theranostic nanosystem design and characterization, in vitro and in vivo analyses, and transcriptome-based gene profiling. In this work, the highly efficient near-infrared II (NIR-II) photoacoustic image (PA)-guided PTT are selectively activated using our developed matrix metalloproteinase (MMP)-triggered in situ assembly of gold nanodandelions (GNDs@gelatin). Unlike other "always-on" NIR PTT agents lacking specific bioactivation and suffering from the intrinsic nonspecific pseudosignals and treatment-related side effects such as metastasis, our GNDs@gelatin possesses important advantages while deployed in cancer PTT that include the following: (1) The theranostic effects could be "turned on" only after specific MMP-2/-9 activity and with acidity in the tumor microenvironment. (2) The quantitative PA diagnosis allows for precise PTT planning for better cancer treatment. (3) GNDs@gelatin could noninvasively quantify MMP activity and efficiently harness NIR-I (808 nm) and NIR-II (1064 nm) energies for tumor ablation. (4) The multibranched nanostructures reabsorb scattered laser photons, thus enhancing the surface plasmons for the pronounced photothermal conversion of aggregated GNDs@gelatin in situ. (5) It is noteworthy that in situ tumor eradication at higher PTT temperature (>55 °C) mediated by GNDs@gelatin could induce subsequent metastasis, which could be otherwise abolished at lower PTT temperatures (50 °C > T > 43 °C). (6) Furthermore, the gene profiling using transcriptome-based microarray including GO and KEGG analyses revealed that 315 differentially expressed genes were identified in higher PTT temperature treated tumors compared with lower PTT temperature ones. These were enriched into some well-known cancer-related pathways, such as cell migration pathway, signal transductions, cell proliferation, wound healing, PPAR signaling, and metabolic pathways. These observations suggest a new perspective of "moderate-is-better" in nanoparticle-mediated PTT for maximizing its therapeutic/prognosis benefits and translational potential with metastasis inhibition.
Keywords: gold nanodandelions; matrix metalloproteinases; metastasis; near-infrared window I and II; photoacoustic imaging; plasmonic photothermal therapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Ultrathin Two-Dimensional Plasmonic PtAg Nanosheets for Broadband Phototheranostics in Both NIR-I and NIR-II Biowindows.Adv Sci (Weinh). 2021 Sep;8(17):e2100386. doi: 10.1002/advs.202100386. Epub 2021 Jul 11. Adv Sci (Weinh). 2021. PMID: 34247445 Free PMC article.
-
A tumor Microenvironment-triggered protein-binding Near-infrared-II Theranostic nanoplatform for Mild-Temperature photothermal therapy.J Colloid Interface Sci. 2025 Feb 15;680(Pt B):375-388. doi: 10.1016/j.jcis.2024.11.049. Epub 2024 Nov 9. J Colloid Interface Sci. 2025. PMID: 39577235
-
Rationally designed dual-plasmonic gold nanorod@cuprous selenide hybrid heterostructures by regioselective overgrowth for in vivo photothermal tumor ablation in the second near-infrared biowindow.Theranostics. 2020 Sep 19;10(25):11656-11672. doi: 10.7150/thno.51287. eCollection 2020. Theranostics. 2020. PMID: 33052239 Free PMC article.
-
Applications of nanotheranostics in the second near-infrared window in bioimaging and cancer treatment.Nanoscale. 2024 Dec 5;16(47):21697-21730. doi: 10.1039/d4nr03058c. Nanoscale. 2024. PMID: 39508492 Review.
-
Second near-infrared photothermal materials for combinational nanotheranostics.Chem Soc Rev. 2021 Jan 21;50(2):1111-1137. doi: 10.1039/d0cs00664e. Epub 2020 Nov 27. Chem Soc Rev. 2021. PMID: 33245316 Review.
Cited by
-
Exosome-mediated triple drug delivery enhances apoptosis in pancreatic cancer cells.Apoptosis. 2025 Aug;30(7-8):1893-1911. doi: 10.1007/s10495-025-02131-y. Epub 2025 Jun 9. Apoptosis. 2025. PMID: 40488835
-
Development of Pd-Loaded Hf-Based Metal-Organic Framework as a Dual-Modal Contrast Agent for Photoacoustic Imaging and Computed Tomography.ACS Biomater Sci Eng. 2025 Jun 9;11(6):3634-3648. doi: 10.1021/acsbiomaterials.5c00169. Epub 2025 May 6. ACS Biomater Sci Eng. 2025. PMID: 40327613 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

