Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study
- PMID: 39046173
- PMCID: PMC11848261
- DOI: 10.1093/cid/ciae384
Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study
Abstract
Background: Semaglutide is highly effective for decreasing weight. Concomitant loss of muscle mass often accompanies weight loss and may have consequences on muscle function.
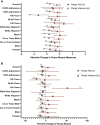
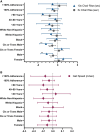
Methods: This is a secondary analysis from the SLIM LIVER (Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections, ACTG A5371) study, a single-arm study of semaglutide in people with human immunodeficiency virus (HIV, PWH) with metabolic dysfunction-associated steatotic liver diseases (MASLD). Participants received subcutaneous semaglutide for 24 weeks (titrated to 1 mg/week by week 4). Psoas volume and fat fraction were assessed from liver magnetic resonance imaging, and physical function was assessed by 10-time chair rise test and 4 m gait speed. Mean change from baseline to week 24 was estimated with linear regression modeling.
Results: Fifty-one PWH were enrolled (muscle measures n = 46). The mean age was 50 years (standard deviation, 11), body mass index was 35.5 kg/m2 (5.6), 43% were women, 33% Black, and 39% Hispanic/Latino. Psoas muscle volume decreased by 9.3% (95% confidence interval [CI]: -13.4 to -5.2; P < .001) over 24 weeks, but psoas muscle fat did not significantly change (-0.42%; 95% CI: -1.00 to .17; P = .16). Chair rise and gait speed showed nonsignificant improvements of 1.27 seconds (95% CI: -2.7 to .10) and 0.05 m/sec (95% CI: -.01 to .10), respectively (both P > .07). The prevalence of slow gait speed (<1 m/sec) decreased from 63% to 46% (P = .029).
Conclusions: In PWH receiving semaglutide for MASLD, despite decreased psoas muscle volume, there was no significant change in physical function, suggesting function was maintained despite significant loss of muscle.
Clinical trials registration: NCT04216589.
Keywords: HIV; function; muscle; obesity; semaglutide.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. J. E. L. serves as a consultant to CytoDyn, Inc. R. M. is the founder and chief technology officer for ForeSpect, PLLC, which served as the imaging core laboratory for this study. T. T. B. has served as a consultant to Gilead Sciences, ViiV Healthcare, Janssen, and Merck. A. L. L. is a consultant for Gilead Sciences and Abbott. K. M. E. receives grant funding from Gilead Sciences and has served as a consultant for ViiV, Gilead, and Merck (all paid to the University of Colorado). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021; 397:2212–24. - PubMed
-
- Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020; 69:1691–705. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

