METTL14-mediated upregulation of lncRNA HOTAIR represses PP1α expression by promoting H3K4me1 demethylation in oxycodone-treated mice
- PMID: 39046182
- PMCID: PMC11267563
- DOI: 10.1111/cns.14830
METTL14-mediated upregulation of lncRNA HOTAIR represses PP1α expression by promoting H3K4me1 demethylation in oxycodone-treated mice
Abstract
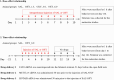
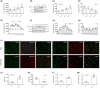
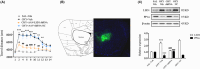
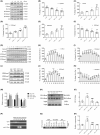
N6-methyladenosine (m6A) methylation is a vital epigenetic mechanism associated with drug addiction. However, the relationship between m6A modification and oxycodone rewarding is less well explored. Based on an open field test, the present study evaluated oxycodone rewarding using chromatin immunoprecipitation PCR, immunofluorescence, and RNA sequencing. A marked increase in METTL14 protein and a decrease in PP1α protein due to oxycodone abundance in the striatal neurons were observed in a dose- and time-dependent manner. Oxycodone markedly increased LSD1 expression, and decreased H3K4me1 expression in the striatum. In the open field test, intra-striatal injection of METTL14 siRNA, HOTAIR siRNA, or LSD1 shRNA blocked oxycodone-induced increase in locomotor activity. The downregulation of PP1α was also inhibited after treatment with METTL14/HOTAIR siRNA and LSD1 shRNA. Enhanced binding of LSD1 with CoRest and of CoRest with the PP1α gene induced by oxycodone was also reversed by LSD1 shRNA. In addition, H3K4me1 demethylation was also blocked by the treatment. In summary, the investigation confirmed that METTL14-mediated upregulation of HOTAIR resulted in the repression of PP1α, which in turn facilitated the recruitment of LSD1, thus catalyzing H3K4me1 demethylation and promoting oxycodone addiction.
Keywords: H3K4me1; LSD1; METTL14; m6A; oxycodone.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

