Advances in the structural basis for angiotensin-1 converting enzyme (ACE) inhibitors
- PMID: 39046229
- PMCID: PMC11300679
- DOI: 10.1042/BSR20240130
Advances in the structural basis for angiotensin-1 converting enzyme (ACE) inhibitors
Abstract
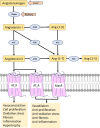
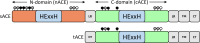
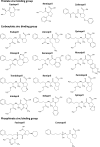
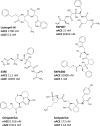
Human somatic angiotensin-converting enzyme (ACE) is a key zinc metallopeptidase that plays a pivotal role in the renin-angiotensin-aldosterone system (RAAS) by regulating blood pressure and electrolyte balance. Inhibition of ACE is a cornerstone in the management of hypertension, cardiovascular diseases, and renal disorders. Recent advances in structural biology techniques have provided invaluable insights into the molecular mechanisms underlying ACE inhibition, facilitating the design and development of more effective therapeutic agents. This review focuses on the latest advancements in elucidating the structural basis for ACE inhibition. High-resolution crystallographic studies of minimally glycosylated individual domains of ACE have revealed intricate molecular details of the ACE catalytic N- and C-domains, and their detailed interactions with clinically relevant and newly designed domain-specific inhibitors. In addition, the recently elucidated structure of the glycosylated form of full-length ACE by cryo-electron microscopy (cryo-EM) has shed light on the mechanism of ACE dimerization and revealed continuous conformational changes which occur prior to ligand binding. In addition to these experimental techniques, computational approaches have also played a pivotal role in elucidating the structural basis for ACE inhibition. Molecular dynamics simulations and computational docking studies have provided atomic details of inhibitor binding kinetics and energetics, facilitating the rational design of novel ACE inhibitors with improved potency and selectivity. Furthermore, computational analysis of the motions observed by cryo-EM allowed the identification of allosteric binding sites on ACE. This affords new opportunities for the development of next-generation allosteric inhibitors with enhanced pharmacological properties. Overall, the insights highlighted in this review could enable the rational design of novel ACE inhibitors with improved efficacy and safety profiles, ultimately leading to better therapeutic outcomes for patients with hypertension and cardiovascular diseases.
Keywords: enzymology; inhibitor design; structural biology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



Similar articles
-
Advances in Structural Biology of ACE and Development of Domain Selective ACE-inhibitors.Med Chem. 2019;15(6):574-587. doi: 10.2174/1573406415666190514081132. Med Chem. 2019. PMID: 31084594 Review.
-
A computational approach to the study of the binding mode of dual ACE/NEP inhibitors.J Chem Inf Model. 2010 Mar 22;50(3):388-96. doi: 10.1021/ci9005047. J Chem Inf Model. 2010. PMID: 20170101
-
The influence of angiotensin converting enzyme mutations on the kinetics and dynamics of N-domain selective inhibition.FEBS J. 2016 Nov;283(21):3941-3961. doi: 10.1111/febs.13900. Epub 2016 Oct 5. FEBS J. 2016. PMID: 27636235
-
Development of domain-selective angiotensin I-converting enzyme inhibitors.Ann N Y Acad Sci. 2005 Nov;1056:160-75. doi: 10.1196/annals.1352.035. Ann N Y Acad Sci. 2005. PMID: 16387685
-
Transforming Non-Selective Angiotensin-Converting Enzyme Inhibitors in C- and N-domain Selective Inhibitors by Using Computational Tools.Mini Rev Med Chem. 2020;20(14):1436-1446. doi: 10.2174/1389557520666191224113830. Mini Rev Med Chem. 2020. PMID: 31889494 Review.
Cited by
-
Finger blood flow (FBF) measurement among vibration-exposed groundskeepers: a pilot study in the southeastern USA.Occup Environ Med. 2025 Apr 16;82(2):83-89. doi: 10.1136/oemed-2024-109979. Occup Environ Med. 2025. PMID: 40185635 Free PMC article.
-
ACE- and DPP-IV-Inhibitory Peptides from Bambara Groundnut Hydrolysate: Elucidation Using Computational Tools and Molecular Docking.Biology (Basel). 2025 May 7;14(5):511. doi: 10.3390/biology14050511. Biology (Basel). 2025. PMID: 40427700 Free PMC article.
-
Dimerization and dynamics of angiotensin-I converting enzyme revealed by cryo-EM and MD simulations.bioRxiv [Preprint]. 2025 Jul 12:2025.01.09.632263. doi: 10.1101/2025.01.09.632263. bioRxiv. 2025. PMID: 39868314 Free PMC article. Preprint.
-
Molecular Basis of Dipeptide Recognition in Drosophila melanogaster Angiotensin I-Converting Enzyme Homologue, AnCE.Biomolecules. 2025 Apr 16;15(4):591. doi: 10.3390/biom15040591. Biomolecules. 2025. PMID: 40305366 Free PMC article.
-
Purification, identification, and in silico screening of a multifunctional octapeptide from semen armeniacae glutelin-2 hydrolysates: restraining mechanisms to Keap1 and ACE, stability, and ferrous-transport efficiency.Front Nutr. 2025 Apr 4;12:1571161. doi: 10.3389/fnut.2025.1571161. eCollection 2025. Front Nutr. 2025. PMID: 40256167 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

