Generalizability in real-world trials
- PMID: 39046315
- PMCID: PMC11267629
- DOI: 10.1111/cts.13886
Generalizability in real-world trials
Abstract
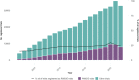
Real-world evidence (RWE) trials have a key advantage over conventional randomized controlled trials (RCTs) due to their potentially better generalizability. High generalizability of study results facilitates new biological insights and enables targeted therapeutic strategies. Random sampling of RWE trial participants is regarded as the gold standard for generalizability. Additionally, the use of sample correction procedures can increase the generalizability of trial results, even when using nonrandomly sampled real-world data (RWD). This study presents descriptive evidence on the extent to which the design of currently planned or already conducted RWE trials takes sampling into account. It also examines whether random sampling or procedures for correcting nonrandom samples are considered. Based on text mining of publicly available metadata provided during registrations of RWE trials on clinicaltrials.gov, EU-PAS, and the OSF-RWE registry, it is shown that the share of RWE trial registrations with information on sampling increased from 65.27% in 2002 to 97.43% in 2022, with a corresponding increase from 14.79% to 28.30% for trials with random samples. For RWE trials with nonrandom samples, there is an increase from 0.00% to 0.95% of trials in which sample correction procedures are used. We conclude that the potential benefits of RWD in terms of generalizing trial results are not yet being fully realized.
© 2024 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
FB reports grants from the Hans Böckler Foundation, the Einstein Foundation, and the Berlin University Alliance; travel expenses covered by the German Society for Anesthesia and Intensive Care Medicine and the Robert Koch Institute; royalties from Elsevier; consulting fees from Medtronic; and honoraria from GE Healthcare, outside of the submitted work. All other authors declared no competing interests in this work.
Figures


References
-
- US Food & Drug Administration . Real‐World Evidence. FDA; 2023. Available from: https://www.fda.gov/science‐research/science‐and‐research‐special‐topics... [accessed Jan 8, 2024]
-
- Näher AF, Vorisek CN, Klopfenstein SA, et al. Secondary data for global health digitalisation. Lancet Digital Health. 2023;5(2):e93‐e101. - PubMed
-
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence—what is it and what can it tell us. N Engl J Med. 2016;375(23):2293‐2297. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

