Anatomy-specific Progression Classification in Chest Radiographs via Weakly Supervised Learning
- PMID: 39046325
- PMCID: PMC11427915
- DOI: 10.1148/ryai.230277
Anatomy-specific Progression Classification in Chest Radiographs via Weakly Supervised Learning
Abstract
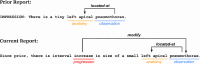
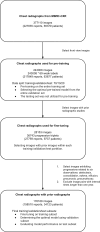
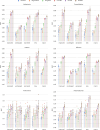
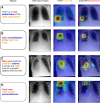
Purpose To develop a machine learning approach for classifying disease progression in chest radiographs using weak labels automatically derived from radiology reports. Materials and Methods In this retrospective study, a twin neural network was developed to classify anatomy-specific disease progression into four categories: improved, unchanged, worsened, and new. A two-step weakly supervised learning approach was employed, pretraining the model on 243 008 frontal chest radiographs from 63 877 patients (mean age, 51.7 years ± 17.0 [SD]; 34 813 [55%] female) included in the MIMIC-CXR database and fine-tuning it on the subset with progression labels derived from consecutive studies. Model performance was evaluated for six pathologic observations on test datasets of unseen patients from the MIMIC-CXR database. Area under the receiver operating characteristic (AUC) analysis was used to evaluate classification performance. The algorithm is also capable of generating bounding-box predictions to localize areas of new progression. Recall, precision, and mean average precision were used to evaluate the new progression localization. One-tailed paired t tests were used to assess statistical significance. Results The model outperformed most baselines in progression classification, achieving macro AUC scores of 0.72 ± 0.004 for atelectasis, 0.75 ± 0.007 for consolidation, 0.76 ± 0.017 for edema, 0.81 ± 0.006 for effusion, 0.7 ± 0.032 for pneumonia, and 0.69 ± 0.01 for pneumothorax. For new observation localization, the model achieved mean average precision scores of 0.25 ± 0.03 for atelectasis, 0.34 ± 0.03 for consolidation, 0.33 ± 0.03 for edema, and 0.31 ± 0.03 for pneumothorax. Conclusion Disease progression classification models were developed on a large chest radiograph dataset, which can be used to monitor interval changes and detect new pathologic conditions on chest radiographs. Keywords: Prognosis, Unsupervised Learning, Transfer Learning, Convolutional Neural Network (CNN), Emergency Radiology, Named Entity Recognition Supplemental material is available for this article. © RSNA, 2024 See also commentary by Alves and Venkadesh in this issue.
Keywords: Convolutional Neural Network (CNN); Emergency Radiology; Named Entity Recognition; Prognosis; Transfer Learning; Unsupervised Learning.
Conflict of interest statement
Figures


Comment in
-
Unveiling Disease Progression in Chest Radiographs through AI.Radiol Artif Intell. 2024 Sep;6(5):e240426. doi: 10.1148/ryai.240426. Radiol Artif Intell. 2024. PMID: 39166968 Free PMC article. No abstract available.
References
-
- Siela D . Chest radiograph evaluation and interpretation . AACN Adv Crit Care 2008. ; 19 ( 4 ): 444 – 473 ; quiz 474–475 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

