Empagliflozin prevents heart failure through inhibition of the NHE1-NO pathway, independent of SGLT2
- PMID: 39046464
- PMCID: PMC11461573
- DOI: 10.1007/s00395-024-01067-9
Empagliflozin prevents heart failure through inhibition of the NHE1-NO pathway, independent of SGLT2
Abstract
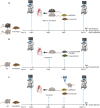
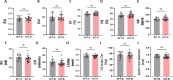
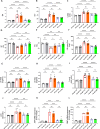
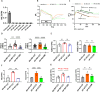
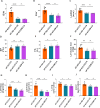
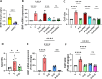
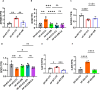
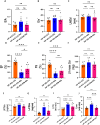
Sodium glucose cotransporter 2 inhibitors (SGLT2i) constitute the only medication class that consistently prevents or attenuates human heart failure (HF) independent of ejection fraction. We have suggested earlier that the protective mechanisms of the SGLT2i Empagliflozin (EMPA) are mediated through reductions in the sodium hydrogen exchanger 1 (NHE1)-nitric oxide (NO) pathway, independent of SGLT2. Here, we examined the role of SGLT2, NHE1 and NO in a murine TAC/DOCA model of HF. SGLT2 knockout mice only showed attenuated systolic dysfunction without having an effect on other signs of HF. EMPA protected against systolic and diastolic dysfunction, hypertrophy, fibrosis, increased Nppa/Nppb mRNA expression and lung/liver edema. In addition, EMPA prevented increases in oxidative stress, sodium calcium exchanger expression and calcium/calmodulin-dependent protein kinase II activation to an equal degree in WT and SGLT2 KO animals. In particular, while NHE1 activity was increased in isolated cardiomyocytes from untreated HF, EMPA treatment prevented this. Since SGLT2 is not required for the protective effects of EMPA, the pathway between NHE1 and NO was further explored in SGLT2 KO animals. In vivo treatment with the specific NHE1-inhibitor Cariporide mimicked the protection by EMPA, without additional protection by EMPA. On the other hand, in vivo inhibition of NOS with L-NAME deteriorated HF and prevented protection by EMPA. In conclusion, the data support that the beneficial effects of EMPA are mediated through the NHE1-NO pathway in TAC/DOCA-induced heart failure and not through SGLT2 inhibition.
Keywords: Diastolic dysfunction; Heart failure; NHE1; Nitric oxide; Oxidative stress; SGLT2 inhibitors.
© 2024. The Author(s).
Conflict of interest statement
PF is the founder and CEO of Pharmahungary Group, a group of R&D companies.
Figures
References
-
- Anker SD, Khan MS, Butler J, Ofstad AP, Peil B, Pfarr E, Doehner W, Sattar N, Coats AJS, Filippatos G, Ferreira JP, Zannad F, Pocock S, Packer M (2023) Weight change and clinical outcomes in heart failure with reduced ejection fraction: insights from EMPEROR-Reduced. Eur J Heart Fail 25:117–127. 10.1002/ejhf.2728 - PMC - PubMed
-
- Baartscheer A, Hardziyenka M, Schumacher CA, Belterman CN, van Borren MM, Verkerk AO, Coronel R, Fiolet JW (2008) Chronic inhibition of the Na+/H+ - exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br J Pharmacol 154:1266–1275. 10.1038/bjp.2008.189 - PMC - PubMed
-
- Baartscheer A, Schumacher CA, van Borren MM, Belterman CN, Coronel R, Fiolet JW (2003) Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res 57:1015–1024. 10.1016/s0008-6363(02)00809-x - PubMed
-
- Bay J, Kohlhaas M, Maack C (2013) Intracellular Na(+) and cardiac metabolism. J Mol Cell Cardiol 61:20–27. 10.1016/j.yjmcc.2013.05.010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

