A candidate reference measurement procedure for quantification of glycocholic acid in human serum based on isotope dilution liquid chromatography-tandem mass spectrometry
- PMID: 39046504
- PMCID: PMC11377629
- DOI: 10.1007/s00216-024-05449-9
A candidate reference measurement procedure for quantification of glycocholic acid in human serum based on isotope dilution liquid chromatography-tandem mass spectrometry
Abstract
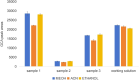
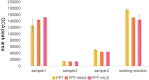
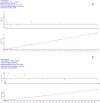
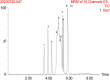
Accurate measurement of serum glycocholic acid (GCA) is crucial for evaluating the activity of chronic hepatitis. Moreover, GCA is a novel identified biomarker for hepatocellular carcinoma. Although some laboratories have used the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to measure GCA in recent years, the problem of potential interference of GCA analogues has not been solved well yet. Neither reference measurement procedures nor reference materials for GCA have been listed in the Joint Committee for Traceability in Laboratory Medicine (JCTLM) database. For standardization of GCA, it is urgent to establish a candidate measurement procedure for GCA. In this study, a candidate reference measurement procedure for the quantification of GCA in human serum based on isotope dilution liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) by a two-step sample pretreatment of protein precipitation and MAX solid-phase extraction was developed and validated. GCA can be completely separated from its structural analogues with gradient elution in 9 min compared with short time gradients published in previous literature by Huang's group. Method validation indicated perfect quantitation precision with intra-day and inter-day values that were ≤1.30% and ≤1.80%, respectively. The method showed excellent linearity with high regression coefficients (R2 > 0.999) over a range of 0.92 ng/g-38.38 μg/g and perfect recoveries at three spiked levels (99.87-100.43%). No interference, matrix effect, and carryover were observed. Moreover, the cRMP was successfully applied to measure GCA in serum samples and compared with two immunoassays in a clinical laboratory. As a candidate reference method, this method can promote a GCA standardization program.
Keywords: Candidate reference measurement procedure; Glycocholic acid; Hepatobiliary disease; Isotope dilution liquid chromatography-tandem mass spectrometry.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Vigne S, Pot C. Implication of oxysterols and phytosterols in aging and human diseases. Implication of oxysterols and phytosterols in aging and human diseases. Cham: Springer; 2023. pp. 231–60. 10.1007/978-3-031-43883-7_12. - PubMed
-
- Glycocholic acid in the Human Metabolome Database. 2017. http://www.hmdb.ca/metabolites/HMDB0000138. Accessed 28 October 2017.
-
- Calton CM, Carothers K, Ramamurthy S, Jagadish N, Phanindra B, Garcia A, et al. Clostridium scindens exacerbates experimental necrotizing enterocolitis via upregulation of the apical sodium-dependent bile acid transporter. Am J Physiol Gastrointest Liver Physiol. 2024;326(1):G25–37. 10.1152/ajpgi.00102.2023. 10.1152/ajpgi.00102.2023 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

