JAK2/mTOR inhibition fails to prevent acute GVHD despite reduced Th1/Th17 cells: final phase 2 trial results
- PMID: 39046783
- PMCID: PMC11619790
- DOI: 10.1182/blood.2024024789
JAK2/mTOR inhibition fails to prevent acute GVHD despite reduced Th1/Th17 cells: final phase 2 trial results
Abstract
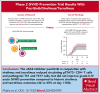
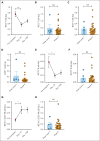
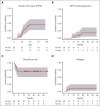
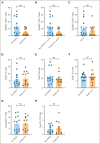
Our phase 1 graft-versus-host disease (GVHD) prevention trial of JAK2 inhibitor, pacritinib (PAC; recommended phase 2 dose: 100 mg orally twice a day on day 0 to +70) plus sirolimus and tacrolimus (SIR/TAC) demonstrated the regimen was safe and free of pan-JAK myelosuppression after allogeneic hematopoietic cell transplantation (alloHCT). PAC inhibits interleukin 6 (IL-6) receptor activity and pathogenic T helper cell 1 (Th1)/Th17 differentiation in preclinical models and the phase 1 trial. Herein, we report on our completed phase 2 trial of PAC/SIR/TAC after 8/8 human leukocyte antigen matched alloHCT. This single-arm phase 2 trial (NCT02891603) was powered to determine if PAC/SIR/TAC suppressed percentage phosphorylated STAT3 (pSTAT3)+ CD4+ T cells at day +21 (primary end point: percentage pSTAT3+ CD4+ T cells ≤ 35%) and estimated grade II to IV acute GVHD by day +100. The impact of PAC/SIR/TAC on T-cell subsets, CD28 (pS6 and pH3ser10), and IL-2 receptor (pSTAT5) signal transduction was also evaluated. Eligible patients (n = 28) received alloHCT for hematologic malignancies or myeloproliferative neoplasms. Reduced or myeloablative intensity conditioning was permitted. PAC/SIR/TAC met the primary end point, reducing percentage pSTAT3+ CD4+ T cells to 9.62% at day +21. Th1/Th17 cells were decreased at day +21, increasing the ratio of regulatory T cells to Th1 and Th17 cells with PAC/SIR/TAC at recommended phase 2 dose PAC compared with dose level 1 PAC. The cumulative incidence of grade II to IV acute GVHD by day +100 with PAC/SIR/TAC was similar to historic SIR/TAC values (46% vs 43%). Although PAC/SIR/TAC suppressed pSTAT3 and Th1/Th17 cells, the regimen did not improve acute GVHD prevention.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.P. reports research support from Novartis, Amgen, Takeda, Janssen, Johnson and Johnson, Pharmacyclics, AbbVie, CTI BioPharma Corp, a Sobi company, and Bristol Myers Squibb; and reports consulting and advisory board membership with Syndax, CTI BioPharma Corp, a Sobi company, Amgen, and Regeneron. S.G.H. reports other support from Incyte and VITRAC Therapeutics. S.G.H. is a clinical trial adjudicator for CSL Behr. B.C.B. reports research support from CTI BioPharma Corp, a Sobi company, VITRAC Therapeutics, and Incyte. B.C.B. holds a patent (WO2015120436A2) related to CD4+ T-cell phosphorylated STAT3 as a marker and therapeutic target of acute graft-versus-host disease (GVHD) and a provisional patent (WO2017058950A1) related to the use of JAK inhibitors for rejection and GVHD prevention. B.C.B. has a patent for WO2019165156 on CD83 chimeric antigen receptor (CAR) T, previously licensed to CRISPR Therapeutics. D.J.W. reports grants from Fate Therapeutics and Incyte outside the submitted work. J.S.M. consults for and holds stock in Fate Therapeutics, GT BioPharma, and Vycellix; and reports research support from Fate Therapeutics and GT Biopharma. J.S.M. serves on the Scientific Advisory Board of ONK Therapeutics, Wugan, and Sanofi. M.L.D. reports grants from CRISPR, Kite/Gilead, and Novartis; other support from Bellicum, Adicet, Adaptive Biotechnologies; and personal fees from Capstan and CARGO. M.L.D. has a patent for CD83 CAR previously licensed to CRISPR. B.R.B. reports research funding from BlueRock Therapeutics and Carisma Therapeutics and consulting fees from BlueRock Therapeutics, Editas Medicine, Janssen Oncology, Sandoz, Legend Biotech, GentiBio Inc, and Magenta Therapeutics. The remaining authors declare no competing financial interests.
Figures



References
-
- Kennedy GA, Varelias A, Vuckovic S, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15(13):1451–1459. - PubMed
-
- Kennedy GA, Tey SK, Buizen L, et al. A phase 3 double-blind study of the addition of tocilizumab vs placebo to cyclosporin/methotrexate GVHD prophylaxis. Blood. 2021;137(14):1970–1979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

