Beneficial Effect of Calcium Treatment for Hyperkalemia Is Not Due to "Membrane Stabilization"
- PMID: 39046789
- PMCID: PMC11410510
- DOI: 10.1097/CCM.0000000000006376
Beneficial Effect of Calcium Treatment for Hyperkalemia Is Not Due to "Membrane Stabilization"
Abstract
Objectives: Hyperkalemia is a common life-threatening condition causing severe electrophysiologic derangements and arrhythmias. The beneficial effects of calcium (Ca 2+ ) treatment for hyperkalemia have been attributed to "membrane stabilization," by restoration of resting membrane potential (RMP). However, the underlying mechanisms remain poorly understood. Our objective was to investigate the mechanisms underlying adverse electrophysiologic effects of hyperkalemia and the therapeutic effects of Ca 2+ treatment.
Design: Controlled experimental trial.
Setting: Laboratory investigation.
Subjects: Canine myocytes and tissue preparations.
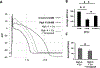
Interventions and measurements: Optical action potentials and volume averaged electrocardiograms were recorded from the transmural wall of ventricular wedge preparations ( n = 7) at baseline (4 mM potassium), hyperkalemia (8-12 mM), and hyperkalemia + Ca 2+ (3.6 mM). Isolated myocytes were studied during hyperkalemia (8 mM) and after Ca 2+ treatment (6 mM) to determine cellular RMP.
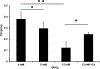
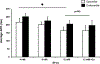
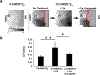
Main results: Hyperkalemia markedly slowed conduction velocity (CV, by 67% ± 7%; p < 0.001) and homogeneously shortened action potential duration (APD, by 20% ± 10%; p < 0.002). In all preparations, this resulted in QRS widening and the "sine wave" pattern observed in severe hyperkalemia. Ca 2+ treatment restored CV (increase by 44% ± 18%; p < 0.02), resulting in narrowing of the QRS and normalization of the electrocardiogram, but did not restore APD. RMP was significantly elevated by hyperkalemia; however, it was not restored with Ca 2+ treatment suggesting a mechanism unrelated to "membrane stabilization." In addition, the effect of Ca 2+ was attenuated during L-type Ca 2+ channel blockade, suggesting a mechanism related to Ca 2+ -dependent (rather than normally sodium-dependent) conduction.
Conclusions: These data suggest that Ca 2+ treatment for hyperkalemia restores conduction through Ca 2+ -dependent propagation, rather than restoration of membrane potential or "membrane stabilization." Our findings provide a mechanistic rationale for Ca 2+ treatment when hyperkalemia produces abnormalities of conduction (i.e., QRS prolongation).
Copyright © 2024 by the Society of Critical Care Medicine and Wolters Kluwer Health, Inc. All Rights Reserved.
Conflict of interest statement
Drs. Piktel, Laurita, and Wilson received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures


References
-
- Littmann L, Gibbs MA: Electrocardiographic manifestations of severe hyperkalemia. J Electrocardiol 2018; 51(5):814–817 - PubMed
-
- Arnsdorf MF: Electrocardiogram in Hyperkalemia: electrocardiographic pattern of anteroseptal myocardial infarction mimicked by hyperkalemia-induced disturbance of impulse conduction. Arch Intern Med 1976; 136(10):1161–1163 - PubMed
-
- Wan X, Bryant SM, Hart G: The effects of [K+]o on regional differences in electrical characteristics of ventricular myocytes in guinea-pig. Exp Physiol 2000; 85(6):769–774 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

