Protocol for assessing GD2 on formalin-fixed paraffin-embedded tissue sections using immunofluorescence staining
- PMID: 39046881
- PMCID: PMC11321291
- DOI: 10.1016/j.xpro.2024.103199
Protocol for assessing GD2 on formalin-fixed paraffin-embedded tissue sections using immunofluorescence staining
Abstract
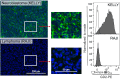
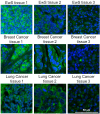
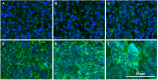
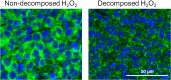
The detection of disialoganglioside GD2 on tumor biopsies, especially in paraffin-embedded tissues, has been challenging due to the glycolipid structure of GD2 and its membrane anchorage. Here, we present an immunofluorescence protocol for the reliable assessment of GD2 on formalin-fixed paraffin-embedded (FFPE) tissues. We describe steps for antigen retrieval with Tris-EDTA buffer and staining with unconjugated anti-GD2 antibody (clone 14.G2a) and horse radish peroxidase (HRP)-conjugated secondary antibody. We then detail procedures for signal amplification using the tyramide signal amplification technique. For complete details on the use and execution of this protocol, please refer to Fischer-Riepe et al.1.
Keywords: antibody; cancer; immunology; microscopy.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Fischer-Riepe L., Kailayangiri S., Zimmermann K., Pfeifer R., Aigner M., Altvater B., Kretschmann S., Volkl S., Hartley J., Dreger C., et al. Preclinical Development of CAR T Cells with Antigen-Inducible IL18 Enforcement to Treat GD2-Positive Solid Cancers. Clin. Cancer Res. 2024:OF1–OF14. doi: 10.1158/1078-0432.CCR-23-3157. - DOI - PubMed
-
- Schulz G., Cheresh D.A., Varki N.M., Yu A., Staffileno L.K., Reisfeld R.A. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44:5914–5920. - PubMed
-
- Ladenstein R., Potschger U., Valteau-Couanet D., Luksch R., Castel V., Yaniv I., Laureys G., Brock P., Michon J.M., Owens C., et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617–1629. doi: 10.1016/S1470-2045(18)30578-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

