Diels-Alder Cycloadditions of Oxacyclic Allenes and α-Pyrones
- PMID: 39046907
- PMCID: PMC11459240
- DOI: 10.1021/acs.orglett.4c02294
Diels-Alder Cycloadditions of Oxacyclic Allenes and α-Pyrones
Abstract
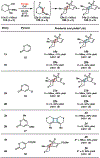
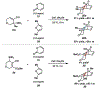
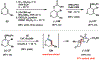
Reactions of α-pyrones with oxacyclic allenes in Diels-Alder trappings are described. We investigate regioselectivity trends and perform competition experiments to assess the influence of structural and electronic features on relative reaction rates. We also demonstrate the stereospecific trapping of an oxacyclic allene, which proceeds in high optical yield. This study provides insight into strained cyclic allene reactivity, as well as new synthetic tools for the rapid construction of complex, heterocyclic scaffolds.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

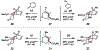
References
-
- Wenk HH; Winkler M; Sander W One century of aryne chemistry. Angew. Chem., Int. Ed 2003, 42, 502–528. - PubMed
-
- Shi J; Li L; Li Y o-Silylaryl triflates: A journey of Kobayashi aryne precursors. Chem. Rev 2021, 121, 3892–4044. - PubMed
-
- Tadross PM; Stoltz BM A comprehensive history of arynes in natural product total synthesis. Chem. Rev 2012, 112, 3550–3577. - PubMed
-
- Gampe CM; Carreira EM Arynes and cyclohexyne in natural product synthesis. Angew. Chem., Int. Ed 2012, 51, 3766–3778. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

