Real-world comparison of health care costs of venetoclax-obinutuzumab vs Bruton's tyrosine kinase inhibitor use among US Medicare beneficiaries with chronic lymphocytic leukemia in the frontline setting
- PMID: 39046941
- PMCID: PMC11424914
- DOI: 10.18553/jmcp.2024.24049
Real-world comparison of health care costs of venetoclax-obinutuzumab vs Bruton's tyrosine kinase inhibitor use among US Medicare beneficiaries with chronic lymphocytic leukemia in the frontline setting
Abstract
Background: Bruton's tyrosine kinase inhibitors (BTKis) and the BCL-2 inhibitor venetoclax in combination with obinutuzumab (VEN-O) are both recommended as frontline therapy in chronic lymphocytic leukemia (CLL). However, VEN-O is a 12-month fixed-duration therapy generating durable remissions whereas BTKis are continuous treat-to-progression treatments.
Objective: To examine costs before and after the fixed-duration treatment period for VEN-O relative to that observed for BTKis in a national sample of older US adults with CLL in the frontline setting.
Methods: This retrospective analysis used Medicare Parts A, B, and D claims from 2016 to 2021. Fee-for-service Medicare beneficiaries aged 66 years or older initiating frontline CLL treatment with VEN-O or a BTKi treatment between June 1, 2019, and June 30, 2020 (index date = first prescription fill date), were included in the sample. Mean cost measures were captured for both groups over 2 fixed time periods calculated from the index date: Month 0 to 12 (proxy for VEN-O on-treatment period) and Month 13 to 18 (proxy for VEN-O off-treatment period). A difference-in-difference approach was used. Multivariate generalized linear models estimated changes in adjusted mean monthly costs during Month 0 to 12 vs Month 13 to 18, for the VEN-O group relative to the BTKi group.
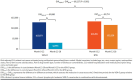
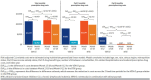
Results: The final sample contained 193 beneficiaries treated with VEN-O and 1,577 beneficiaries treated with BTKis. Risk-adjusted all-cause monthly total costs were similar for VEN-O patients ($13,887) and BTKi patients ($14,492) between Month 0 and 12. Moreover, during Month 13 to 18, the mean monthly all-cause total costs declined by 67% for VEN-O ($13,887 to $4,462) but only by 10% for BTKi ($14,492 to $13,051). Hence, the relative reduction in costs across the 2 periods was significantly larger for VEN-O (-$9,425) vs BTKi (-$1,441) patients (ie, difference in difference = -$7,984; P < 0.001). Similar patterns were observed for CLL-related costs, with the substantially larger reductions in CLL-related total monthly costs (-$9,880 VEN-O vs -$1,753 BTKi; P < 0.001) for the VEN-O group primarily driven by the larger reduction in CLL-related monthly prescription costs (-$9,437 VEN-O vs -$2,020 BTKi; P < 0.001).
Conclusions: This real-world study of older adults with CLL found a large reduction in monthly Medicare costs in the 6 months after completion of the fixed-duration treatment period of VEN-O, largely driven by the reduction in CLL-related prescription drug costs. A similar decline in costs was not observed among those treated with BTKis. Our study highlights the substantial economic benefits of fixed-duration VEN-O relative to treat-to-progression therapies like BTKis in the first-line CLL setting.
Conflict of interest statement
Drs Puckett and Kamal-Bahl are full-time employees of COVIA Health Solutions, a consulting firm with clients in the biotech/pharmaceutical industry. Dr Manzoor, Ms Jawaid, and Dr Emechebe are employees of AbbVie Inc. and may hold stock or stock options. Ms Ravelo is an employee of Genentech Inc. and may hold stock or stock options. Dr Huntington reports consultancy for AbbVie, Janssen, Genentech, Flatiron Health, BeiGene, AstraZeneca, ADC Therapeutics, Epizyme, Merck, Seattle Genetics, TG Therapeutics, Thyme, Pharmacyclics, SeaGen, and Arvinas; research funding from Celgene, DTRM Biopharm, and TG Therapeutics; and honoraria from Pharmacyclics, AstraZeneca, and Bayer. Dr Doshi reports consultancy for AbbVie, Acadia, Janssen, Merck, Otsuka, and Takeda and research funding from Janssen, Merck, and Spark Therapeutics.
Figures


References
-
- Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679-705. doi:10.1002/ajh.26367 - PubMed
-
- Stauder R, Eichhorst B, Hamaker ME, et al. . Management of chronic lymphocytic leukemia (CLL) in the elderly: a position paper from an international Society of Geriatric Oncology (SIOG) Task Force. Ann Oncol. 2017;28(2):218-27. doi:10.1093/annonc/mdw547 - PubMed
-
- Stilgenbauer S. Prognostic markers and standard management of chronic lymphocytic leukemia. Hematology (Am Soc Hematol Educ Program). 2015;2015(1):368-77. doi:10.1182/asheducation-2015.1.368 - PubMed
-
- Iovino L, Shadman M. Novel therapies in chronic lymphocytic leukemia: A rapidly changing landscape. Curr Treat Options Oncol. 2020;21(4):24. doi:10.1007/s11864-020-0715-5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

