Combined metabolomics and transcriptomics reveal the secondary metabolite networks in different growth stages of Bletilla striata (Thunb.) Reichb.f
- PMID: 39046970
- PMCID: PMC11290943
- DOI: 10.1371/journal.pone.0307260
Combined metabolomics and transcriptomics reveal the secondary metabolite networks in different growth stages of Bletilla striata (Thunb.) Reichb.f
Abstract
Background: Bletilla striata (Thunb.) Reichb.f. (B. striata) is a traditional Chinese medicinal herb. B. striata polysaccharides (BSP), stilbenes and 2-isobutyl malic acid glucosoxy-benzyl ester compounds are the main active ingredients in B. striata. However, there is limited report on the changes of medicinal components and their biosynthesis regulation mechanisms in the tubers of B. striata at different stages.
Method: The tubers of B. striata were collected during the flowering period, fruiting period, and harvest period to determine the total polysaccharide content using the phenol sulfuric acid method. The changes in secondary metabolites in the tubers at these stages were analyzed by ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS), and transcriptomics was conducted for further exploration of their biosynthetic pathways.
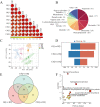
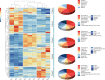
Result: The BSP content gradually increases from the flowering period to the fruiting period as the tubers develop, reaching its peak, but subsequently decreases at harvest time, which may be associated with the germination of B. striata buds in later stage. A total of 294 compounds were identified in this study. Among them, a majority of the compounds, such as 2-isobutyl malate gluconoxy-benzyl ester, exhibited high content during the fruit stage, while stilbenes like coelonin, 3'-O-methylbatatasin III, and blestriarene A accumulated during the harvesting period. The transcriptome data also revealed a substantial number of differentially expressed genes at various stages, providing a partial explanation for the complex changes in metabolites. We observed a correspondence between the expression pattern of GDP-Man biosynthesis-related enzyme genes and cumulative changes in BSP. And identified a positive correlation between 9 transcription factors and genes associated with polysaccharide biosynthesis, while 5 transcription factors were positively correlated with accumulation of 2-isobutyl malate gluconoxy-benzyl ester compounds and 5 transcription factors exhibited negative correlated with stilbene accumulation.
Conclusion: It is imperative to determine the appropriate harvesting period based on the specific requirements of different active ingredients and the accumulation patterns of their metabolites. Considering the involvement of multiple transcription factors in the biosynthesis and accumulation of its active ingredients, a comprehensive investigation into the specific regulatory mechanisms that facilitate high-quality cultivation of B. striata is imperative.
Copyright: © 2024 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- Zhu Z, Liang T, Dai G, Zheng J, Dong J, Xia C, et al.. Extraction, structural-activity relationships, bioactivities, and application prospects of Bletilla striata polysaccharides as ingredients for functional products: A review. Int J Biol Macromol. 2023;245: 125407. doi: 10.1016/j.ijbiomac.2023.125407 - DOI - PubMed
-
- Sun L, Yu Z, Li H, Li J, Liu H, Lin X, et al.. Advances in Secondary Metabolites of Medicinal Plant. Journal of jilin agriculyural sciences. 2009;34: 4–10.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

