Double-stranded RNA induces antiviral transcriptional response through the Dicer-2/Ampk/FoxO axis in an arthropod
- PMID: 39047046
- PMCID: PMC11295077
- DOI: 10.1073/pnas.2409233121
Double-stranded RNA induces antiviral transcriptional response through the Dicer-2/Ampk/FoxO axis in an arthropod
Abstract
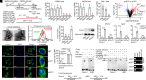
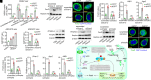
Invertebrates mainly rely on sequence-specific RNA interference (RNAi) to resist viral infections. Increasing studies show that double-stranded RNA (dsRNA) can induce sequence-independent protection and that Dicer-2, the key RNAi player that cleaves long dsRNA into small interfering RNA (siRNA), is necessary for this protection. However, how this protection occurs remains unknown. Herein, we report that it is caused by adenosine triphosphate (ATP)-hydrolysis accompanying the dsRNA-cleavage. Dicer-2 helicase domain is ATP-dependent; therefore, the cleavage consumes ATP. ATP depletion activates adenosine monophosphate-activated protein kinase (Ampk) and induces nuclear localization of Fork head box O (FoxO), a key transcriptional factor for dsRNA-induced genes. siRNAs that do not require processing cannot activate the transcriptional response. This study reveals a unique nonspecific antiviral mechanism other than the specific RNAi in shrimp. This mechanism is functionally similar to, but mechanistically different from, the dsRNA-activated antiviral response in vertebrates and suggests an interesting evolution of innate antiviral immunity.
Keywords: Dicer-2; RNA-interference; double-stranded RNA.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Labreuche Y., et al. , Non-specific activation of antiviral immunity and induction of RNA interference may engage the same pathway in the Pacific white leg shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 34, 1209–1218 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

