Dietary and water restriction leads to increased susceptibility to antimicrobial resistant pathogens
- PMID: 39047095
- PMCID: PMC11268424
- DOI: 10.1126/sciadv.adi7438
Dietary and water restriction leads to increased susceptibility to antimicrobial resistant pathogens
Abstract
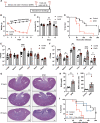
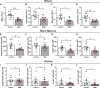
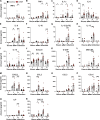
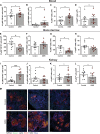
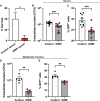
Dehydration and malnutrition are common and often underdiagnosed in hospital settings. Multidrug-resistant bacterial infections result in more than 35,000 deaths a year in nosocomial patients. The effect of temporal dietary and water restriction (DWR) on susceptibility to multidrug-resistant pathogens is unknown. We report that DWR markedly increased susceptibility to systemic infection by ESKAPE pathogens. Using a murine bloodstream model of methicillin-resistant Staphylococcus aureus infection, we show that DWR leads to significantly increased mortality and morbidity. DWR causes increased bacterial burden, severe pathology, and increased numbers of phagocytes in the kidney. DWR appears to alter the functionality of these phagocytes and is therefore unable to control infection. Mechanistically, we show that DWR impairs the ability of macrophages to phagocytose multiple bacterial pathogens and efferocytose apoptotic neutrophils. Together, this work highlights the crucial impact that diet and hydration play in protecting against infection.
Figures



References
-
- Silver H. J., Pratt K. J., Bruno M., Lynch J., Mitchell K., McCauley S. M., Effectiveness of the malnutrition quality improvement initiative on practitioner malnutrition knowledge and screening, diagnosis, and timeliness of malnutrition-related care provided to older adults admitted to a tertiary care facility: A pilot study. J. Acad. Nutr. Diet. 118, 101–109 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

