Macrophage depletion protects against cisplatin-induced ototoxicity and nephrotoxicity
- PMID: 39047106
- PMCID: PMC11268410
- DOI: 10.1126/sciadv.adk9878
Macrophage depletion protects against cisplatin-induced ototoxicity and nephrotoxicity
Abstract
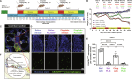
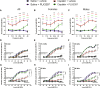
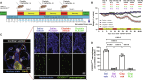
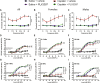
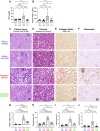
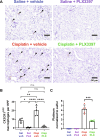
Cisplatin is a widely used anticancer drug with notable side effects including ototoxicity and nephrotoxicity. Macrophages, the major resident immune cells in the cochlea and kidney, are important drivers of both inflammatory and tissue repair responses. To investigate the roles of macrophages in cisplatin-induced toxicities, we used PLX3397, a U.S. Food and Drug Administration-approved inhibitor of the colony-stimulating factor 1 receptor, to eliminate tissue-resident macrophages. Mice treated with cisplatin alone had considerable hearing loss (ototoxicity) and kidney injury (nephrotoxicity). Macrophage ablation resulted in significantly reduced hearing loss and had greater outer hair cell survival. Macrophage ablation also protected against cisplatin-induced nephrotoxicity, as evidenced by markedly reduced tubular injury and fibrosis. Mechanistically, our data suggest that the protective effect of macrophage ablation against cisplatin-induced ototoxicity and nephrotoxicity is mediated by reduced platinum accumulation in both the inner ear and the kidney. Together, our data indicate that ablation of tissue-resident macrophages represents an important strategy for mitigating cisplatin-induced ototoxicity and nephrotoxicity.
Figures



Update of
-
Macrophage Depletion Protects Against Cisplatin-Induced Ototoxicity and Nephrotoxicity.bioRxiv [Preprint]. 2023 Nov 17:2023.11.16.567274. doi: 10.1101/2023.11.16.567274. bioRxiv. 2023. Update in: Sci Adv. 2024 Jul 26;10(30):eadk9878. doi: 10.1126/sciadv.adk9878. PMID: 38014097 Free PMC article. Updated. Preprint.
References
-
- Frisina R. D., Wheeler H. E., Fossa S. D., Kerns S. L., Fung C., Sesso H. D., Monahan P. O., Feldman D. R., Hamilton R., Vaughn D. J., Beard C. J., Budnick A., Johnson E. M., Ardeshir-Rouhani-Fard S., Einhorn L. H., Lipshultz S. E., Dolan M. E., Travis L. B., Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J. Clin. Oncol. 34, 2712–2720 (2016). - PMC - PubMed
-
- Rademaker-Lakhai J. M., Crul M., Zuur L., Baas P., Beijnen J. H., Simis Y. J. W., van Zandwijk N., Schellens J. H. M., Relationship between cisplatin administration and the development of ototoxicity. J. Clin. Oncol. 24, 918–924 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

