Relapsing-Remitting Immunotherapy Responsive Small-Fiber Neuropathy: Longitudinal Tracking Through 10 Years Including Pregnancies
- PMID: 39047208
- PMCID: PMC11270892
- DOI: 10.1212/NXI.0000000000200286
Relapsing-Remitting Immunotherapy Responsive Small-Fiber Neuropathy: Longitudinal Tracking Through 10 Years Including Pregnancies
Abstract
Objectives: To expand understanding of the pathogenesis, presentations, and treatment of initially idiopathic small fiber polyneuropathy (SFN).
Methods: We longitudinally readministered validated metrics to track disease course and treatment responses in a previously healthy woman with acute, postinfectious, skin biopsy-confirmed, idiopathic SFN.
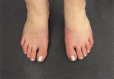
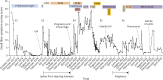
Results: During 5 years, viral respiratory infections triggered 3 separated episodes of acute, disabling burning hand, foot, and face pain (erythromelalgia). The initial 2 resolved with high-dose prednisone, and the third responded to repeated immunoglobulin treatments. Pregnancy with miscarriage triggered a fourth exacerbation refractory to corticosteroids and cyclosporin. Immunoglobulins restored total remission for 2 months; then, 2 rituximab doses slightly improved later flaring. Subsequently, daratumumab initiated 100-day remission later maintained by belimumab, initiated to permit another pregnancy. Remission continued after gestational week 13 all-treatment withdrawal. A week 30 fifth flare responded to plasmapheresis, with healthy birth at week 40. At 11-week postpartum, as symptoms returned, restarting belimumab restored remission maintained during ≥19 months of breastfeeding.
Discussion: This decade of tracking characterizes a relapsing-remitting course of SFN with initially separated monophasic episodes becoming more confluent, as with multiple sclerosis. This tempo and responsiveness to 5 immunotherapies suggest dysimmune causality. Validated metrics helped define the course and track treatment efficacy, particularly during pregnancy and breastfeeding.
Classification of evidence: This is a single observational study without controls. This provides Class IV evidence.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures
Similar articles
-
Acute monophasic erythromelalgia pain in five children diagnosed as small-fiber neuropathy.Eur J Paediatr Neurol. 2020 Sep;28:198-204. doi: 10.1016/j.ejpn.2020.06.004. Epub 2020 Jul 7. Eur J Paediatr Neurol. 2020. PMID: 32723684 Free PMC article.
-
Small Fiber Neuropathy in Children: Two Case Reports Illustrating the Importance of Recognition.Pediatrics. 2016 Oct;138(4):e20161215. doi: 10.1542/peds.2016-1215. Pediatrics. 2016. PMID: 27660061
-
Intravenous Immunoglobulin Therapy in Patients With Painful Idiopathic Small Fiber Neuropathy.Neurology. 2021 May 18;96(20):e2534-e2545. doi: 10.1212/WNL.0000000000011919. Epub 2021 Mar 25. Neurology. 2021. PMID: 33766992 Free PMC article. Clinical Trial.
-
Immunotherapy Prospects for Painful Small-fiber Sensory Neuropathies and Ganglionopathies.Neurotherapeutics. 2016 Jan;13(1):108-17. doi: 10.1007/s13311-015-0395-1. Neurotherapeutics. 2016. PMID: 26526686 Free PMC article. Review.
-
Advances in the Management of Small Fiber Neuropathy.Neurol Clin. 2021 Feb;39(1):113-131. doi: 10.1016/j.ncl.2020.09.006. Epub 2020 Nov 7. Neurol Clin. 2021. PMID: 33223078 Review.
Cited by
-
Diagnostic challenges of long COVID in children: a survey of pediatric health care providers' preferences and practices.Front Pediatr. 2024 Dec 23;12:1484941. doi: 10.3389/fped.2024.1484941. eCollection 2024. Front Pediatr. 2024. PMID: 39764158 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
