FOXP4 Is a Direct YAP1 Target That Promotes Gastric Cancer Stemness and Drives Metastasis
- PMID: 39047223
- PMCID: PMC11532785
- DOI: 10.1158/0008-5472.CAN-23-3074
FOXP4 Is a Direct YAP1 Target That Promotes Gastric Cancer Stemness and Drives Metastasis
Abstract
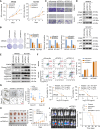
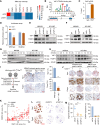
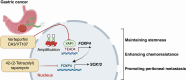
The Hippo-YAP1 pathway is an evolutionally conserved signaling cascade that controls organ size and tissue regeneration. Dysregulation of Hippo-YAP1 signaling promotes initiation and progression of several types of cancer, including gastric cancer. As the Hippo-YAP1 pathway regulates expression of thousands of genes, it is important to establish which target genes contribute to the oncogenic program driven by YAP1 to identify strategies to circumvent it. In this study, we identified a vital role of forkhead box protein 4 (FOXP4) in YAP1-driven gastric carcinogenesis by maintaining stemness and promoting peritoneal metastasis. Loss of FOXP4 impaired gastric cancer spheroid formation and reduced stemness marker expression, whereas FOXP4 upregulation potentiated cancer cell stemness. RNA sequencing analysis revealed SOX12 as a downstream target of FOXP4, and functional studies established that SOX12 supports stemness in YAP1-induced carcinogenesis. A small-molecule screen identified 42-(2-tetrazolyl) rapamycin as a FOXP4 inhibitor, and targeting FOXP4 suppressed gastric cancer tumor growth and enhanced the efficacy of 5-fluorouracil chemotherapy in vivo. Collectively, these findings revealed that FOXP4 upregulation by YAP1 in gastric cancer regulates stemness and tumorigenesis by upregulating SOX12. Targeting the YAP1-FOXP4-SOX12 axis represents a potential therapeutic strategy for gastric cancer. Significance: Hippo-YAP1 signaling maintains stemness in gastric cancer by upregulating FOXP4, identifying FOXP4 as a stemness biomarker and therapeutic target that could help improve patient outcomes.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
K.W. Lo reports grants from Viracta Therapeutics and ScinnoHub Pharmaceutical Co., Ltd., outside the submitted work. No disclosures were reported by the other authors.
Figures





References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–11. - PubMed
-
- Saygin C, Matei D, Majeti R, Reizes O, Lathia JD. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell 2019;24:25–40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

