Levodopa: From Biological Significance to Continuous Monitoring
- PMID: 39047295
- PMCID: PMC11348912
- DOI: 10.1021/acssensors.4c00602
Levodopa: From Biological Significance to Continuous Monitoring
Abstract
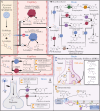
A continuous levodopa sensor can improve the quality of life for patients suffering with Parkinson's disease by enhancing levodopa titration and treatment effectiveness; however, its development is currently hindered by the absence of a specific levodopa molecular recognition element and limited insights into how real-time monitoring might affect clinical outcomes. This gap in research contributes to clinician uncertainty regarding the practical value of continuous levodopa monitoring data. This paper examines the current state of levodopa sensing and the inherent limitations in today's methods. Further, these challenges are described, including aspects such as interference from the metabolic pathway and adjunct medications, temporal resolution, and clinical questions, with a specific focus on a comprehensive selection of molecules, such as adjunct medications and structural isomers, as an interferent panel designed to assess and validate future levodopa sensors. We review insights and lessons from previously reported levodopa sensors and present a comparative analysis of potential molecular recognition elements, discussing their advantages and drawbacks.
Keywords: Parkinson’s disease; and sensor specificity; biosensor development; continuous levodopa monitoring; electrochemical detection; levodopa therapy; molecular recognition elements.
Conflict of interest statement
The authors declare the following competing financial interest(s): K.B., D.P., and K.S. are inventors of three patents related to this work filed by the University of North Carolina and Chapel Hill. (1) U.S. Provisional Patent Application No. 63/505,267, filed May 31, 2023, (2) U.S. Provisional Patent Application No. 63/505,267, filed May 31, 2023, and (3) U.S. Provisional Patent Application No. 63/505,259, filed May 31, 2023. The authors declare no other competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

