Heme oxygenase-1 protects cells from replication stress
- PMID: 39047636
- PMCID: PMC11321372
- DOI: 10.1016/j.redox.2024.103247
Heme oxygenase-1 protects cells from replication stress
Abstract
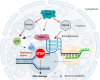
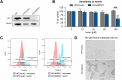
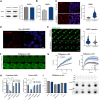
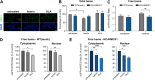
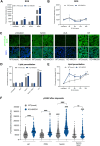
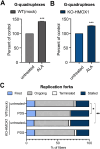
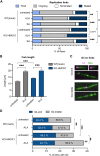
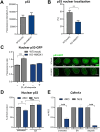
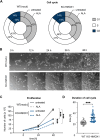
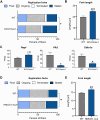
Heme oxygenase-1 (HO-1, HMOX1) degrades heme protecting cells from heme-induced oxidative damage. Beyond its well-established cellular functions, heme has emerged as a stabilizer of G-quadruplexes. These secondary DNA structures interfere with DNA replication. We recently revealed that nuclear HO-1 colocalizes with DNA G-quadruplexes and promotes their removal. Here, we investigate whether HO-1 safeguards cells against replication stress. Experiments were conducted in control and HMOX1-deficient HEK293T cell lines. Immunostaining unveiled that DNA G-quadruplexes accumulated in the absence of HO-1, the effect that was further enhanced in response to δ-aminolevulinic acid (ALA), a substrate in heme synthesis. This was associated with replication stress, as evidenced by an elevated proportion of stalled forks analyzed by fiber assay. We observed the same effects in hematopoietic stem cells isolated from Hmox1 knockout mice and in a lymphoblastoid cell line from an HMOX1-deficient patient. Interestingly, in the absence of HO-1, the speed of fork progression was higher, and the response to DNA conformational hindrance less stringent, indicating dysfunction of the PARP1-p53-p21 axis. PARP1 activity was not decreased in the absence of HO-1. Instead, we observed that HO-1 deficiency impairs the nuclear import and accumulation of p53, an effect dependent on the removal of excess heme. We also demonstrated that administering ALA is a more specific method for increasing intracellular free heme compared to treatment with hemin, which in turn induces strong lipid peroxidation. Our results indicate that protection against replication stress is a universal feature of HO-1, presumably contributing to its widely recognized cytoprotective activity.
Keywords: Cell cycle; G-quadruplexes; Heme; Heme oxygenase-1; PARP1; Replication stress.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Li C., Wu J., Dong Q., et al. Heme oxygenase-1 impacts oxidative stress-induced neural stem cells senescence by regulating DNA damage repair via PARP-1. SSRN. 2023 Preprint.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

