Tanycytic transcytosis inhibition disrupts energy balance, glucose homeostasis and cognitive function in male mice
- PMID: 39047908
- PMCID: PMC11340606
- DOI: 10.1016/j.molmet.2024.101996
Tanycytic transcytosis inhibition disrupts energy balance, glucose homeostasis and cognitive function in male mice
Abstract
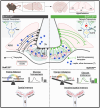
Objectives: In Western society, high-caloric diets rich in fats and sugars have fueled the obesity epidemic and its related disorders. Disruption of the body-brain communication, crucial for maintaining glucose and energy homeostasis, arises from both obesogenic and genetic factors, leading to metabolic disorders. Here, we investigate the role of hypothalamic tanycyte shuttles between the pituitary portal blood and the third ventricle cerebrospinal fluid in regulating energy balance.
Methods: We inhibited vesicle-associated membrane proteins (VAMP1-3)-mediated release in tanycytes by expressing the botulinum neurotoxin type B light chain (BoNT/B) in a Cre-dependent manner in tanycytes. This was achieved by injecting either TAT-Cre in the third ventricle or an AAV1/2 expressing Cre under the control of the tanycyte-specific promoter iodothyronine deiodinase 2 into the lateral ventricle of adult male mice.
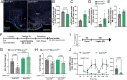
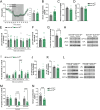
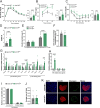
Results: In male mice fed a standard diet, targeted expression of BoNT/B in adult tanycytes blocks leptin transport into the mediobasal hypothalamus and results in normal-weight central obesity, including increased food intake, abdominal fat deposition, and elevated leptin levels but no marked change in body weight. Furthermore, BoNT/B expression in adult tanycytes promotes fatty acid storage, leading to glucose intolerance and insulin resistance. Notably, these metabolic disturbances occur despite a compensatory increase in insulin secretion, observed both in response to exogenous glucose boluses in vivo and in isolated pancreatic islets. Intriguingly, these metabolic alterations are associated with impaired spatial memory in BoNT/B-expressing mice.
Conclusions: These findings underscore the central role of tanycytes in brain-periphery communication and highlight their potential implication in the age-related development of type 2 diabetes and cognitive decline. Our tanycytic BoNT/B mouse model provides a robust platform for studying how these conditions progress over time, from prediabetic states to full-blown metabolic and cognitive disorders, and the mechanistic contribution of tanycytes to their development. The recognition of the impact of tanycytic transcytosis on hormone transport opens new avenues for developing targeted therapies that could address both metabolic disorders and their associated cognitive comorbidities, which often emerge or worsen with advancing age.
Keywords: Blood-cerebrospinal fluid barrier; Blood–brain barrier; Hypothalamus; Normal-weight central obesity; Tanycytes; Transports.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. - PubMed
-
- Lustig R.H. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nature clinical practice. Endocrinology & metabolism. 2006;2:447–458. - PubMed
-
- Mathieu C., Martens P.J., Vangoitsenhoven R. One hundred years of insulin therapy. Nat Rev Endocrinol. 2021;17(12):715–725. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

