Neural circuit basis of placebo pain relief
- PMID: 39048016
- PMCID: PMC11358037
- DOI: 10.1038/s41586-024-07816-z
Neural circuit basis of placebo pain relief
Abstract
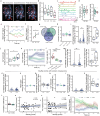
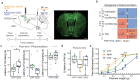
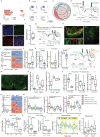
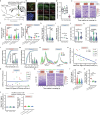
Placebo effects are notable demonstrations of mind-body interactions1,2. During pain perception, in the absence of any treatment, an expectation of pain relief can reduce the experience of pain-a phenomenon known as placebo analgesia3-6. However, despite the strength of placebo effects and their impact on everyday human experience and the failure of clinical trials for new therapeutics7, the neural circuit basis of placebo effects has remained unclear. Here we show that analgesia from the expectation of pain relief is mediated by rostral anterior cingulate cortex (rACC) neurons that project to the pontine nucleus (rACC→Pn)-a precerebellar nucleus with no established function in pain. We created a behavioural assay that generates placebo-like anticipatory pain relief in mice. In vivo calcium imaging of neural activity and electrophysiological recordings in brain slices showed that expectations of pain relief boost the activity of rACC→Pn neurons and potentiate neurotransmission in this pathway. Transcriptomic studies of Pn neurons revealed an abundance of opioid receptors, further suggesting a role in pain modulation. Inhibition of the rACC→Pn pathway disrupted placebo analgesia and decreased pain thresholds, whereas activation elicited analgesia in the absence of placebo conditioning. Finally, Purkinje cells exhibited activity patterns resembling those of rACC→Pn neurons during pain-relief expectation, providing cellular-level evidence for a role of the cerebellum in cognitive pain modulation. These findings open the possibility of targeting this prefrontal cortico-ponto-cerebellar pathway with drugs or neurostimulation to treat pain.
© 2024. The Author(s).
Conflict of interest statement
During part of this project, M.J.S. was a consultant for and had a financial interest in Inscopix, the company that makes the miniature microscope used for Ca2+ imaging in this study.
Figures









References
-
- Harrington, A. The Cure Within: A History of Mind-Body Medicine (W. W. Norton & Company, 2008).
-
- Putnam, H. The Threefold Cord: Mind, Body, and World (Columbia Univ. Press, 1999).
-
- Bingel, U. et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl. Med.3, 70ra14 (2011). - PubMed
-
- Colloca, L. & Benedetti, F. Placebo analgesia induced by social observational learning. Pain144, 28–34 (2009). - PubMed
-
- Fields, H. L. How expectations influence pain. Pain159, S3–S10 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

