Accumulation of alkyl-lysophosphatidylcholines in Niemann-Pick disease type C1
- PMID: 39048052
- PMCID: PMC11367646
- DOI: 10.1016/j.jlr.2024.100600
Accumulation of alkyl-lysophosphatidylcholines in Niemann-Pick disease type C1
Abstract
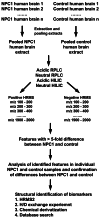
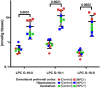
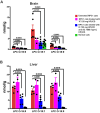
Lysosomal function is impaired in Niemann-Pick disease type C1 (NPC1), a rare and inherited neurodegenerative disorder, resulting in late endosomal/lysosomal accumulation of unesterified cholesterol. The precise pathogenic mechanism of NPC1 remains incompletely understood. In this study, we employed metabolomics to uncover secondary accumulated substances in NPC1. Our findings unveiled a substantial elevation in the levels of three alkyl-lysophosphatidylcholine [alkyl-LPC, also known as lyso-platelet activating factor (PAF)] species in NPC1 compared to controls across various tissues, including brain tissue from individuals with NPC1, liver, spleen, cerebrum, cerebellum, and brain stem from NPC1 mice, as well as in both brain and liver tissue from NPC1 cats. The three elevated alkyl-LPC species were as follows: LPC O-16:0, LPC O-18:1, and LPC O-18:0. However, the levels of PAF 16:0, PAF 18:1, and PAF 18:0 were not altered in NPC1. In the NPC1 feline model, the brain and liver alkyl-LPC levels were reduced following 2-hydroxypropyl-β-cyclodextrin (HPβCD) treatment, suggesting that alkyl-LPCs are secondary storage metabolites in NPC1 disease. Unexpectedly, cerebrospinal fluid (CSF) levels of LPC O-16:0 and LPC O-18:1 were decreased in individuals with NPC1 compared to age-appropriate comparison samples, and their levels were increased in 80% of participants 2 years after intrathecal HPβCD treatment. The fold increases in CSF LPC O-16:0 and LPC O-18:1 levels were more pronounced in responders compared to nonresponders. This study identified alkyl-LPC species as secondary storage metabolites in NPC1 and indicates that LPC O-16:0 and LPC O-18:1, in particular, could serve as potential biomarkers for tracking treatment response in NPC1 patients.
Keywords: Niemann-Pick disease type C; alkyl-lysophosphatidylcholine; biomarker; mass spectrometry; structural identification.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Xuntian Jiang is an Editorial Board Member of Journal of Lipid Research. The other author declares that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Runz H., Dolle D., Schlitter A.M., Zschocke J. NPC-db, a Niemann-Pick type C disease gene variation database. Hum. Mutat. 2008;29:345–350. - PubMed
-
- Vanier M.T. Lipid changes in Niemann-Pick disease type C brain: personal experience and review of the literature. Neurochem. Res. 1999;24:481–489. - PubMed
-
- Vanier M.T. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis. 2015;38:187–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

