Nondestructive flash cathode recycling
- PMID: 39048568
- PMCID: PMC11269590
- DOI: 10.1038/s41467-024-50324-x
Nondestructive flash cathode recycling
Abstract
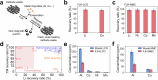
Effective recycling of end-of-life Li-ion batteries (LIBs) is essential due to continuous accumulation of battery waste and gradual depletion of battery metal resources. The present closed-loop solutions include destructive conversion to metal compounds, by destroying the entire three-dimensional morphology of the cathode through continuous thermal treatment or harsh wet extraction methods, and direct regeneration by lithium replenishment. Here, we report a solvent- and water-free flash Joule heating (FJH) method combined with magnetic separation to restore fresh cathodes from waste cathodes, followed by solid-state relithiation. The entire process is called flash recycling. This FJH method exhibits the merits of milliseconds of duration and high battery metal recovery yields of ~98%. After FJH, the cathodes reveal intact core structures with hierarchical features, implying the feasibility of their reconstituting into new cathodes. Relithiated cathodes are further used in LIBs, and show good electrochemical performance, comparable to new commercial counterparts. Life-cycle-analysis highlights that flash recycling has higher environmental and economic benefits over traditional destructive recycling processes.
© 2024. The Author(s).
Conflict of interest statement
Rice University owns intellectual property on the flash recycling process disclosed here. That intellectual property is licensed to a company in which JMT is a shareholder, but he is not an officer, director or employee. Conflicts of interest are mitigated through compliance with the Rice University Office of Research Integrity. The other authors claim no current conflicts of interest.
Figures




References
-
- Rey, I., Vallejo, C., Santiago, G., Iturrondobeitia, M. & Lizundia, E. Environmental impacts of graphite recycling from spent lithium-ion batteries based on life cycle assessment. ACS Sustain. Chem. Eng.9, 14488–14501 (2021). 10.1021/acssuschemeng.1c04938 - DOI
-
- Li, H. et al. A contact-electro-catalytic cathode recycling method for spent lithium-ion batteries. Nat. Energy8, 1137–1144 (2023). 10.1038/s41560-023-01348-y - DOI
-
- Martínez, O. V., Valio, J., Aarnio, A. S., Reuter, M. & Guerrero, R. S. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries5, 68 (2019). 10.3390/batteries5040068 - DOI
-
- Jacoby, M. It’s time to get serious about recycling lithium-ion batteries. Chem. Eng. Newshttps://cen.acs.org/materials/energy-storage/time-serious-recycling-lith... (2020).
Grants and funding
- FA9550-19-1-029/United States Department of Defense | United States Air Force | AFMC | Air Force Office of Scientific Research (AF Office of Scientific Research)
- W912HZ-21-2-0050/United States Department of Defense | United States Army | US Army Corps of Engineers | Engineer Research and Development Center (U.S. Army Engineer Research and Development Center)
LinkOut - more resources
Full Text Sources

