Identification of new correctors for traffic-defective ABCB4 variants by a high-content screening approach
- PMID: 39048674
- PMCID: PMC11269752
- DOI: 10.1038/s42003-024-06590-y
Identification of new correctors for traffic-defective ABCB4 variants by a high-content screening approach
Abstract
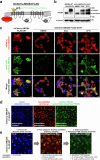
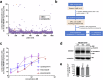
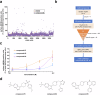
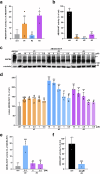
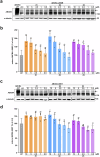
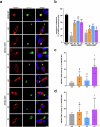
ABCB4 is located at the canalicular membrane of hepatocytes and is responsible for the secretion of phosphatidylcholine into bile. Genetic variations of this transporter are correlated with rare cholestatic liver diseases, the most severe being progressive familial intrahepatic cholestasis type 3 (PFIC3). PFIC3 patients most often require liver transplantation. In this context of unmet medical need, we developed a high-content screening approach to identify small molecules able to correct ABCB4 molecular defects. Intracellularly-retained variants of ABCB4 were expressed in cell models and their maturation, cellular localization and function were analyzed after treatment with the molecules identified by high-content screening. In total, six hits were identified by high-content screening. Three of them were able to correct the maturation and canalicular localization of two distinct intracellularly-retained ABCB4 variants; one molecule was able to significantly restore the function of two ABCB4 variants. In addition, in silico molecular docking calculations suggest that the identified hits may interact with wild type ABCB4 residues involved in ATP binding/hydrolysis. Our results pave the way for their optimization in order to provide new drug candidates as potential alternative to liver transplantation for patients with severe forms of ABCB4-related diseases, including PFIC3.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Smit, J. J. et al. Tissue distribution of the human MDR3 P-glycoprotein. Lab Invest71, 638–649 (1994). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- ANR-15-CE14-0008-01/Agence Nationale de la Recherche (French National Research Agency)
- ANR-19-CE17-0020-01/Agence Nationale de la Recherche (French National Research Agency)
- DIGPHAT 22-PESN-0017/Agence Nationale de la Recherche (French National Research Agency)
- ANR-21-CE18-0030-01/Agence Nationale de la Recherche (French National Research Agency)
- ANR-10-EQPX-04-01/Agence Nationale de la Recherche (French National Research Agency)
LinkOut - more resources
Full Text Sources

