Pembrolizumab monotherapy survival benefits in metastatic non-small-cell lung cancer: a systematic review of real-world data
- PMID: 39048812
- PMCID: PMC11269554
- DOI: 10.1007/s12672-024-01153-3
Pembrolizumab monotherapy survival benefits in metastatic non-small-cell lung cancer: a systematic review of real-world data
Abstract
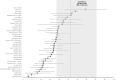
The efficacy of pembrolizumab in the treatment-naïve non-small-cell lung cancer (NSCLC) patients was proved in the KEYNOTE-024 randomized trial. The aim of this systematic literature review was to identify and summarize the real world evidence (RWE) of overall survival (OS) in previously untreated patients with NSCLC receiving pembrolizumab monotherapy. A systematic search was conducted in PubMed (MEDLINE®) and EMBASE databases. Analyses were focused on survival data (median OS and survival rates at specific time points). To explore the population comparable with the KEYNOTE-024 study, we focused on studies enrolling at least 50% of patients at stage IV of cancer and ECOG performance status 0-2. A total of 41 RWE studies covering over 7600 advanced NSCLC patients naïve to systemic treatment were identified. Overall, survival outcomes reported in those studies vary considerably (median OS range: 3.0-34.6 months). Most RWE studies reported median OS shorter to that reported in KEYNOTE-024 (26.3 months), but about half of reported OS medians were in range of 95% confidence interval for OS as reported in KEYNOTE-024 trial (18.3-40.4 months). Patients with similar characteristics of stage and performance status to those of KEYNOTE-024 trial benefited the same with pembrolizumab monotherapy as their survival outcomes (18.9-22.8 months) were consistent with those reported in the clinical trial. RWE data showed substantially worse outcomes in patients with ECOG-PS 2+ compared to ECOG-PS 0-1 patients.
Keywords: Monotherapy; Non-small-cell lung cancer; Overall survivor; Pembrolizumab; Performance status; Real world evidence.
© 2024. The Author(s).
Conflict of interest statement
HealthQuest is the health technology assessment consultancy supporting pharma manufacturers in reimbursement application and preparation of HTA dossiers. Professor Maciej Krzakowski declares conflict of interest in a form of honoraria for lectures and advisory boards from MSD, ROCHE, BMS, ASTRAZENECA.
Figures




References
-
- World Health Organization. Cancer, key facts. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 22 Sept 2022.
-
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192-iv237. 10.1093/annonc/mdy275. Erratum in: Ann Oncol. 2019;30(5):863–870, https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-1.... Accessed 22 Sept 2022. - PubMed
-
- https://www.ema.europa.eu/en/documents/product-information/keytruda-epar.... Accessed 22 Sept 2022.
Publication types
LinkOut - more resources
Full Text Sources
