Extrafine single inhaler triple therapy effectiveness in COPD patients previously treated with multiple-inhaler triple therapy: the TRIWIN study
- PMID: 39049587
- PMCID: PMC11301738
- DOI: 10.1177/17534666241263439
Extrafine single inhaler triple therapy effectiveness in COPD patients previously treated with multiple-inhaler triple therapy: the TRIWIN study
Abstract
Background: The extrafine single inhaler triple therapy (efSITT) containing beclomethasone dipropionate/formoterol fumarate/glycopyrronium 87/5/9 μg has proved to be efficacious in patients with chronic obstructive pulmonary disease (COPD) in randomized control trials.
Objective: TRIWIN study evaluated the effectiveness of efSITT delivering beclomethasone dipropionate/formoterol fumarate/glycopyrronium 87/5/9 μg in COPD patients previously treated with multiple-inhaler triple therapy (MITT) in a real-world study in Greece.
Design: Prospective, multicenter, observational, non-interventional study was conducted over 24 weeks.
Methods: A total of 475 eligible patients had moderate-to-severe COPD, an indication for treatment with efSITT, and were symptomatic despite receiving MITT. COPD Assessment Test (CAT) score, pulmonary function parameters, use of rescue medication, and adherence to inhaler use were recorded at baseline (Visit 1), 3 (Visit 2), and 6 months (Visit 3) after treatment.
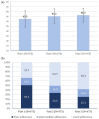
Results: Mean CAT score decreased from 21.4 points at Visit 1, to 16.6 at Visit 2 and 15.1 at Visit 3 (p < 0.001 for all pair comparisons). At Visit 3, 79.8% of patients reached a CAT improvement exceeding minimal clinically important difference (⩾2), compared to baseline. Mean forced expiratory volume in 1 s (%pred.) increased from 55.4% at Visit 1 to 63.5% at the end of study period (p < 0.001), while mean forced vital capacity (%pred.) increased from 71.1% at Visit 1, to 76.7% at Visit 3 (p < 0.001). The mean Test of Adherence to Inhalers score increased from 42.5 to 45.3 and 46.3 points, for the three visits, respectively (p < 0.001 comparing Visits 1/2 and Visits 1/3; p = 0.006 comparing Visits 2/3). The percentage of patients showing good adherence rose from 33.7% at baseline to 58.3% at Visit 3. The percentage of patients using rescue medication during the last month dropped from 16.2% to 7.4% at the end of study period (p < 0.001). Pulmonary function parameters also improved.
Conclusion: The TRIWIN results suggest that extrafine beclomethasone dipropionate/formoterol fumarate/glycopyrronium is effective in improving health status, pulmonary function, and adherence and in reducing rescue medication use in COPD patients previously treated with MITT, in a real-world setting in Greece.
Keywords: COPD; extrafine; health status; multiple-inhaler triple therapy; real-world study; single inhaler triple therapy; treatment adherence.
Conflict of interest statement
PS reports honoraria from Astrazeneca, Chiesi, GSK, ELPEN, Menarini, and Novartis and consultancy fees from Astrazeneca, Chiesi, GSK, ELPEN, Menarini, Novartis, and Specialty Therapeutics. NG reports honoraria from Astrazeneca, Menarini, Boehringer, GSK, and MSD, consultancy fees from: Astrazeneca, Novartis, and Menarini, fees for participation in clinical trials from Astrazeneca, Boehringer Ingelheim, Chiesi, Menarini, Novartis, and GSK, as well as coverage of participation in national and international congresses. GK reports honoraria and consultancy fees from Astrazeneca, Chiesi, Elpen, GSK, Menarini, Novartis, Pharmaten, and Sanofi. KB reports honoraria and consultancy fees from: Astrazeneca, Chiesi, ELPEN, GSK, Menarini, and Novartis. AK was employed by Chiesi Hellas during this study execution, manuscript preparation, and submission and is currently an Astrazeneca Greece employee. PE and ST are employees of Chiesi Hellas S.A. PK reports financial support as statistical analyst of the present study. DP reports consultancy fees from: Chiesi, ELPEN, Innovis, Menarini, and Roche, honoraria from: Astrazeneca and Menarini, coverage of participation costs (registration, travel, and accommodation) in national and international congresses from: Astrazeneca, Bayer, Boehringer Ingelheim, Chiesi, ELPEN, Lilly, Menarini, Novartis, Pfizer, and participation in clinical studies of Amgen, Astrazeneca, Boehringer Ingelheim, Chiesi, ELPEN, GSK, Menarini, Novartis, and Sanofi. KP reports honoraria and consultancy fees from: Astrazeneca, Boehringer Ingelheim, Chiesi, CSL Behring, ELPEN, Innovis, GSK, Menarini, Novartis, Pharmathen, Sanofi, Specialty Therapeutics, and UCB.
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease. 2023. Report, https://goldcopd.org/2023-gold-report-2/ (accessed 14 July 2023).
-
- World Health Organization. Chronic obstructive pulmonary disease (COPD), https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pul... (accessed 14 July 2023).
-
- Celli BR, Thomas NE, Anderson JA, et al.. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008; 178: 332–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

