Anti-TNF therapy impairs both short- and long-term IgG responses after repeated vaccination
- PMID: 39049686
- PMCID: PMC11804311
- DOI: 10.1111/all.16241
Anti-TNF therapy impairs both short- and long-term IgG responses after repeated vaccination
Abstract
Background: Recently, it has been questioned whether vaccination of patients with inflammatory (auto)immune diseases under anti-tumor necrosis factor (TNF) treatment leads to impaired vaccine-induced immune responses and protection against breakthrough infections. However, the effects of TNF blockade on short- and long-term immune responses after repeated vaccination remain unclear. Vaccination studies have shown that initial short-term IgG antibodies (Abs) carry highly galactosylated and sialylated Fc glycans, whilst long-term IgG Abs have low levels of galactosylation and sialylation and are most likely generated by long-lived plasma cells (PCs) derived primarily from the germinal center (GC) response. Thus, IgG Fc glycosylation patterns may be applicable to distinguish short- and long-term vaccine responses after repeated vaccination under the influence of anti-TNF treatment.
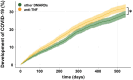
Methods: We used COVID-19 vaccination as a model to investigate vaccine-induced IgG subclass levels and Fc glycosylation patterns, B cell subsets, and effector functions of short- and long-term Ab responses after up to three vaccinations in patients on anti-TNF or other immunosuppressive treatments and in healthy individuals. Using TriNetX, a global healthcare database, we determined the risk of SARS-CoV-2 breakthrough infections in vaccinated patients treated with anti-TNF or other immunosuppressive drugs.
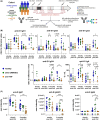
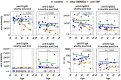
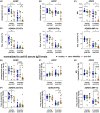
Results: Anti-TNF treatment reduced the long-term abundance of all anti-S IgG subclasses with low levels of galactosylation and sialylation. Re-activation of potential memory B cells initially generated highly galactosylated and sialylated IgG antibodies, which were progressively reduced after each booster dose in anti-TNF-treated patients, especially in the elderly. The reduced short- and long-term IgG (1) levels in anti-TNF-treated patients correlated with diminished functional activity and an increased risk for the development of COVID-19.
Conclusions: The data suggest that anti-TNF treatment reduces both GC-dependent long-lived PCs and GC-dependent memory B cell-derived short-lived PCs, hence both the long- and short-term IgG subclass responses, respectively, after repeated vaccination. We propose that anti-TNF therapy, especially in the elderly, reduces the benefit of booster vaccination.
Keywords: COVID‐19; IgG; IgG glycosylation; IgG4; SARS‐CoV‐2; TriNetX; antibody; anti‐TNF treatment; germinal center; inflammatory diseases; long‐lived plasma cell; mRNA vaccine; memory B cell; short‐lived plasma cell; vaccination.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

