Early protein delivery in critically ill patients with acute kidney injury: post hoc analysis of a multicenter cluster-randomized controlled trial
- PMID: 39049866
- PMCID: PMC11267585
- DOI: 10.1093/burnst/tkae027
Early protein delivery in critically ill patients with acute kidney injury: post hoc analysis of a multicenter cluster-randomized controlled trial
Abstract
Background: There is controversy over the optimal early protein delivery in critically ill patients with acute kidney injury (AKI). This study aims to evaluate whether the association between early protein delivery and 28-day mortality was impacted by the presence of AKI in critically ill patients.
Methods: This is a post hoc analysis of data from a multicenter cluster-randomised controlled trial enrolling newly admitted critically ill patients (n = 2772). Participants without chronic kidney disease and with complete data concerning baseline renal function were included in this study. The primary outcome was 28-day mortality. Cox proportional hazards models were used to analyze the association between early protein delivery, reflected by mean protein delivery from day 3-5 after enrollment, 28-day mortality and whether baseline AKI stages interacted with this association.
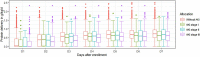
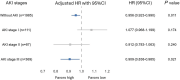
Results: Overall, 2552 patients were included, among whom 567 (22.2%) had AKI at enrollment (111 stage I, 87 stage II, 369 stage III). Mean early protein delivery was 0.60 ± 0.38 g/kg/day among the study patients. In the overall study cohort, each 0.1 g/kg/day increase in protein delivery was associated with a 5% reduction in 28-day mortality[hazard ratio (HR) = 0.95; 95% confidence interval (CI) 0.92-0.98, p < 0.001]. The association between early protein delivery and 28-day mortality significantly interacted with baseline AKI stages (adjusted interaction p = 0.028). Each 0.1 g/kg/day increase in early protein delivery was associated with a 4% reduction in 28-day mortality (HR = 0.96; 95%CI 0.92-0.99, p = 0.011) among patients without AKI and 9% (HR = 0.91; 95%CI 0.84-0.99, p = 0.021) among those with AKI stage III. However, such associations cannot be observed among patients with AKI stages I and II.
Conclusions: Increased early protein delivery (up to close to the guideline recommendation) was associated with reduced 28-day mortality in critically ill patients without AKI and with AKI stage III, but not in those with AKI stage I or II.
Keywords: Acute kidney injury; Critical illness; Mortality; Protein delivery; Renal replacement therapy.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
ARHvZ reports receiving honoraria for advisory board meetings, lectures and research, and travel expenses from Abbott, AOP Pharma, Baxter, Cardinal Health, Danone-Nutricia, DIM3, Fresenius Kabi, GE Healthcare, InBody, Mermaid, Nestle, PAION, Rousselot and Lyric. LK reports grants from Nutricia Pharmaceutical (Wuxi) Co., Ltd China, and personal fees from SciClone Pharmaceuticals. The remaining authors declare no conflict of interest.
Figures

References
-
- Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–600. - PubMed
-
- Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. 2023;42:1671–89. - PubMed
-
- Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2022;46:12–41. - PubMed
LinkOut - more resources
Full Text Sources

