Predicting Chemical Immunotoxicity through Data-Driven QSAR Modeling of Aryl Hydrocarbon Receptor Agonism and Related Toxicity Mechanisms
- PMID: 39049897
- PMCID: PMC11264268
- DOI: 10.1021/envhealth.4c00026
Predicting Chemical Immunotoxicity through Data-Driven QSAR Modeling of Aryl Hydrocarbon Receptor Agonism and Related Toxicity Mechanisms
Abstract
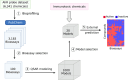
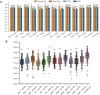
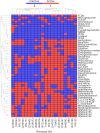
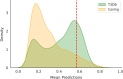
Computational modeling has emerged as a time-saving and cost-effective alternative to traditional animal testing for assessing chemicals for their potential hazards. However, few computational modeling studies for immunotoxicity were reported, with few models available for predicting toxicants due to the lack of training data and the complex mechanisms of immunotoxicity. In this study, we employed a data-driven quantitative structure-activity relationship (QSAR) modeling workflow to extensively enlarge the limited training data by revealing multiple targets involved in immunotoxicity. To this end, a probe data set of 6,341 chemicals was obtained from a high-throughput screening (HTS) assay testing for the activation of the aryl hydrocarbon receptor (AhR) signaling pathway, a key event leading to immunotoxicity. Searching this probe data set against PubChem yielded 3,183 assays with testing results for varying proportions of these 6,341 compounds. 100 assays were selected to develop QSAR models based on their correlations to AhR agonism. Twelve individual QSAR models were built for each assay using combinations of four machine-learning algorithms and three molecular fingerprints. 5-fold cross-validation of the resulting models showed good predictivity (average CCR = 0.73). A total of 20 assays were further selected based on QSAR model performance, and their resulting QSAR models showed good predictivity of potential immunotoxicants from external chemicals. This study provides a computational modeling strategy that can utilize large public toxicity data sets for modeling immunotoxicity and other toxicity endpoints, which have limited training data and complicated toxicity mechanisms.
© 2024 The Authors. Co-published by Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, and American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Data-Driven Quantitative Structure-Activity Relationship Modeling for Human Carcinogenicity by Chronic Oral Exposure.Environ Sci Technol. 2023 Apr 25;57(16):6573-6588. doi: 10.1021/acs.est.3c00648. Epub 2023 Apr 11. Environ Sci Technol. 2023. PMID: 37040559 Free PMC article.
-
Hybrid non-animal modeling: A mechanistic approach to predict chemical hepatotoxicity.J Hazard Mater. 2024 Jun 5;471:134297. doi: 10.1016/j.jhazmat.2024.134297. Epub 2024 Apr 12. J Hazard Mater. 2024. PMID: 38677119 Free PMC article.
-
Predictive Modeling of Estrogen Receptor Binding Agents Using Advanced Cheminformatics Tools and Massive Public Data.Front Environ Sci. 2016 Mar;4:12. doi: 10.3389/fenvs.2016.00012. Epub 2016 Mar 8. Front Environ Sci. 2016. PMID: 27642585 Free PMC article.
-
From QSAR to QSIIR: searching for enhanced computational toxicology models.Methods Mol Biol. 2013;930:53-65. doi: 10.1007/978-1-62703-059-5_3. Methods Mol Biol. 2013. PMID: 23086837 Free PMC article. Review.
-
QSAR: Using the Past to Study the Present.Methods Mol Biol. 2025;2834:3-39. doi: 10.1007/978-1-0716-4003-6_1. Methods Mol Biol. 2025. PMID: 39312158 Review.
Cited by
-
Overview of Computational Toxicology Methods Applied in Drug and Green Chemical Discovery.J Xenobiot. 2024 Dec 4;14(4):1901-1918. doi: 10.3390/jox14040101. J Xenobiot. 2024. PMID: 39728409 Free PMC article. Review.
References
-
- U.S. Congress ; Office of Technology Assessment. Identifying and Controlling Immunotoxic Substances - Background Paper, OTA-BP-BA-75; U.S. Government Printing Office: Washington, DC, 1991.
-
- Descotes J.Chapter 3: Health Consequences of Immunotoxic Effects. In Principles and Methods of Immunotoxicology; Elsevier: The Netherlands, 1986.
-
- Krewski D.; Acosta D.; Andersen M.; Anderson H.; Bailar J. C.; Boekelheide K.; Brent R.; Charnley G.; Cheung V. G.; Green S.; Kelsey K. T.; Kerkvliet N. I.; Li A. A.; McCray L.; Meyer O.; Patterson R. D.; Pennie W.; Scala R. A.; Solomon G. M.; Stephens M.; Yager J.; Zeise L.; et al. Staff of Committee on Toxicity Testing and Assessment of Environmental Agents. Toxicity Testing in the 21st Century: A Vision and a Strategy. J. Toxicol. Environ. Health Part B 2010, 13 (2–4), 51–138. 10.1080/10937404.2010.483176. - DOI - PMC - PubMed
-
- Lankveld D. P. K.; Van Loveren H.; Baken K. A.; Vandebriel R. J. In Vitro Testing for Direct Immunotoxicity: State of the Art. In Immunotoxicity Testing: Methods and Protocols; Dietert R. R., Ed.; Methods in Molecular Biology; Humana: Totowa, NJ, 2010; pp 401–423. 10.1007/978-1-60761-401-2_26. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
