Regulation of bone homeostasis: signaling pathways and therapeutic targets
- PMID: 39049966
- PMCID: PMC11266958
- DOI: 10.1002/mco2.657
Regulation of bone homeostasis: signaling pathways and therapeutic targets
Abstract
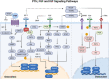
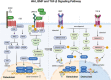
As a highly dynamic tissue, bone is continuously rebuilt throughout life. Both bone formation by osteoblasts and bone resorption by osteoclasts constitute bone reconstruction homeostasis. The equilibrium of bone homeostasis is governed by many complicated signaling pathways that weave together to form an intricate network. These pathways coordinate the meticulous processes of bone formation and resorption, ensuring the structural integrity and dynamic vitality of the skeletal system. Dysregulation of the bone homeostatic regulatory signaling network contributes to the development and progression of many skeletal diseases. Significantly, imbalanced bone homeostasis further disrupts the signaling network and triggers a cascade reaction that exacerbates disease progression and engenders a deleterious cycle. Here, we summarize the influence of signaling pathways on bone homeostasis, elucidating the interplay and crosstalk among them. Additionally, we review the mechanisms underpinning bone homeostatic imbalances across diverse disease landscapes, highlighting current and prospective therapeutic targets and clinical drugs. We hope that this review will contribute to a holistic understanding of the signaling pathways and molecular mechanisms sustaining bone homeostasis, which are promising to contribute to further research on bone homeostasis and shed light on the development of targeted drugs.
Keywords: bone cells; bone homeostasis; signal crosstalk; signaling pathway; skeletal disease; therapeutic targets.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cho E, Chen Z, Ding M, et al. PMSA prevents osteoclastogenesis and estrogen‐dependent bone loss in mice. Bone. 2021;142:115707. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
