Efficacy of sodium hypochlorite in overcoming antimicrobial resistance and eradicating biofilms in clinical pathogens from pressure ulcers
- PMID: 39050624
- PMCID: PMC11266179
- DOI: 10.3389/fmicb.2024.1432883
Efficacy of sodium hypochlorite in overcoming antimicrobial resistance and eradicating biofilms in clinical pathogens from pressure ulcers
Abstract
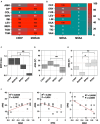
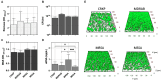
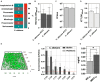
Sodium hypochlorite (NaOCl) is widely recognized for its broad-spectrum antimicrobial efficacy in skin wound care. This study investigates the effectiveness of NaOCl against a range of bacterial and fungal isolates from pressure ulcer (PU) patients. We analyzed 20 bacterial isolates from PU patients, comprising carbapenem-resistant Klebsiella pneumoniae (CRKP), multidrug-resistant Acinetobacter baumannii (MDRAB), methicillin-resistant Staphylococcus aureus (MRSA), methicillin-susceptible Staphylococcus aureus (MSSA), along with 5 Candida albicans isolates. Antibiotic resistance profiles were determined using standard susceptibility testing. Whole-genome sequencing (WGS) was employed to identify antimicrobial resistance genes (ARGs) and disinfectant resistance genes (DRGs). Genetic determinants of biofilm formation were also assessed. The antimicrobial activity of NaOCl was evaluated by determining the minimum inhibitory concentration (MIC) and the minimal biofilm eradication concentration (MBEC) for both planktonic and biofilm-associated cells. CRKP and MDRAB showed resistance to fluoroquinolones and carbapenems, while MRSA exhibited resistance to β-lactams and levofloxacin. MSSA displayed a comparatively lower resistance profile. WGS identified significant numbers of ARGs in CRKP and MDRAB, with fewer DRGs compared to MRSA and MSSA. All isolates possessed genes associated with fimbriae production and adhesion, correlating with pronounced biofilm biomass production. NaOCl demonstrated substantial antimicrobial activity against both planktonic cells and biofilms. The MIC90 for planktonic bacterial cells was 0.125 mg/mL, and the MBEC90 ranged from 0.225 to 0.5 mg/mL. For planktonic C. albicans, the MIC90 was 0.150 mg/mL, and the MBEC90 was 0.250 mg/mL. These results highlight the challenge in treating biofilm-associated infections and underscore the potential of NaOCl as a robust antimicrobial agent against difficult-to-treat biofilm infections at concentrations lower than those typically found in commercial disinfectants.
Keywords: Acinetobacter baumannii; Candida albicans; ESKAPE; Klebsiella pneumoniae; NaOCl; Staphylococcus aureus; biofilm; skin; sodium hypochlorite solution.
Copyright © 2024 Fabrizio, Sivori, Cavallo, Truglio, Toma, Sperati, Francalancia, Obregon, Pamparau, Kovacs, Pimpinelli and Di Domenico.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Alcock B. P., Huynh W., Chalil R., Smith K. W., Raphenya A. R., Wlodarski M. A., et al. . (2023). CARD 2023: expanded curation, support for machine learning, and resistome prediction at the comprehensive antibiotic resistance database. Nucleic Acids Res. 51, D690–D699. doi: 10.1093/nar/gkac920, PMID: - DOI - PMC - PubMed
-
- Binsuwaidan R., Khan M. A., Alzahrani R. H., Aldusaymani A. M., Almallouhi N. M., Alsabti A. S., et al. . (2023). Prevalence of multidrug-resistant and ESBL-producing bacterial pathogens in patients with chronic wound infections and spinal cord injury admitted to a tertiary care rehabilitation hospital. Antibiotics 12:1587. doi: 10.3390/antibiotics12111587, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

