A discrete-to-continuum model for the human cornea with application to keratoconus
- PMID: 39050729
- PMCID: PMC11265872
- DOI: 10.1098/rsos.240265
A discrete-to-continuum model for the human cornea with application to keratoconus
Abstract
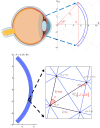
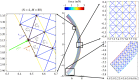
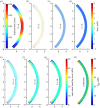
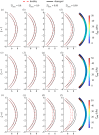
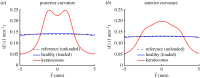
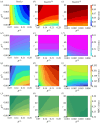
We introduce a discrete mathematical model for the mechanical behaviour of a planar slice of human corneal tissue, in equilibrium under the action of physiological intraocular pressure (IOP). The model considers a regular (two-dimensional) network of structural elements mimicking a discrete number of parallel collagen lamellae connected by proteoglycan-based chemical bonds (crosslinks). Since the thickness of each collagen lamella is small compared to the overall corneal thickness, we upscale the discrete force balance into a continuum system of partial differential equations and deduce the corresponding macroscopic stress tensor and strain energy function for the micro-structured corneal tissue. We demonstrate that, for physiological values of the IOP, the predictions of the discrete model converge to those of the continuum model. We use the continuum model to simulate the progression of the degenerative disease known as keratoconus, characterized by a localized bulging of the corneal shell. We assign a spatial distribution of damage (i.e. reduction of the stiffness) to the mechanical properties of the structural elements and predict the resulting macroscopic shape of the cornea, showing that a large reduction in the element stiffness results in substantial corneal thinning and a significant increase in the curvature of both the anterior and posterior surfaces.
Keywords: collagen lamellae; corneal mechanics; discrete-to-continuum asymptotics; keratoconus; multiscale modelling; proteoglycan matrix.
© 2024 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
-
- Wang J, Liu X, Bao F, Lopes BT, Wang L, Eliasy A, Abass A, Elsheikh A. 2021. Review of ex-vivo characterisation of corneal biomechanics. Med. Nov. Technol. Dev. 11, 100074. (10.1016/j.medntd.2021.100074) - DOI
Associated data
LinkOut - more resources
Full Text Sources

