Risk factors for non-previa placenta accreta spectrum in pregnancies conceived through frozen embryo transfer during a hormone replacement cycle in Japan
- PMID: 39050787
- PMCID: PMC11266119
- DOI: 10.1002/rmb2.12592
Risk factors for non-previa placenta accreta spectrum in pregnancies conceived through frozen embryo transfer during a hormone replacement cycle in Japan
Abstract
Purpose: Non-previa placenta accreta spectrum (PAS) is associated with assisted reproductive technology (ART), particularly frozen embryo transfer during hormone replacement therapy (HRC-FET). We especially aimed to evaluate the prevalence and risk factors for non-previa PAS in HRC-FET pregnancies.
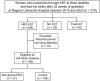
Methods: Overall, 279 women who conceived through ART at three ART facilities and delivered at a single center were included in this retrospective study. Data regarding endometrial thickness at embryo transfer, previous histories, and type of embryo transfer-HRC-FET, frozen embryo transfer during a natural ovulatory cycle (NC-FET), and fresh embryo transfer (Fresh-ET)-were collected. Univariable logistic regression analyses were conducted.
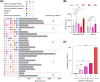
Results: The prevalence of non-previa PAS was 27/192 (14.1%) in the HRC-FET group and 0 (0.0%) in both the NC-FET and Fresh-ET groups. Significantly high odds ratio [95% confidence interval] of non-previa PAS was associated with a history of artificial abortion (6.45 [1.98-21.02]), endometrial thickness <8.0 mm (6.11 [1.06-35.12]), resolved low-lying placenta (5.73 [2.13-15.41]), multiparity (2.90 [1.26-6.69]), polycystic ovarian syndrome (2.62 [1.02-6.71]), and subchorionic hematoma (2.49 [1.03-6.04]).
Conclusions: A history of artificial abortion, endometrial thickness <8.0 mm, and resolved low-lying placenta may help in antenatal detection of a high-risk population of non-previa PAS in HRC-FET pregnancies.
Keywords: assisted reproductive technology; frozen embryo transfer; hormone replacement cycle; non‐previa placenta accreta spectrum; placenta accreta spectrum.
© 2024 The Author(s). Reproductive Medicine and Biology published by John Wiley & Sons Australia, Ltd on behalf of Japan Society for Reproductive Medicine.
Conflict of interest statement
Authors declare no conflict of interests for this article.
Figures

References
-
- Bhide A. Routine screening for placenta accreta spectrum. Best Pract Res Clin Obstet Gynaecol. 2023;90:102392. - PubMed
-
- D'Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2013;42:509–517. - PubMed
-
- Committee on Obstetric Practice . Committee opinion no. 529: placenta accreta. Obstet Gynecol. 2012;120:207–211. - PubMed
-
- Matsuzaki S, Mandelbaum RS, Sangara RN, McCarthy LE, Vestal NL, Klar M, et al. Trends, characteristics, and outcomes of placenta accreta spectrum: a national study in the United States. Am J Obstet Gynecol. 2021;225:534.e1–e38. - PubMed
LinkOut - more resources
Full Text Sources
