n-Bu4NI/K2S2O8-MEDIATED C-N COUPLING BETWEEN ALDEHYDES AND AMIDES
- PMID: 39051029
- PMCID: PMC11268833
- DOI: 10.1002/ejoc.202400067
n-Bu4NI/K2S2O8-MEDIATED C-N COUPLING BETWEEN ALDEHYDES AND AMIDES
Abstract
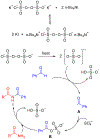
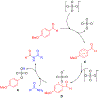
n-Bu4NI/K2S2O8 mediated C-N coupling between aldehydes and amides is reported. A strong electronic effect is observed on the aromatic aldehyde substrates. The transformylation from aldehyde to amide takes place exclusively when an aromatic aldehyde bears electron-donating groups at either the ortho or para position of the formyl group, while the cross-dehydrogenative coupling dominates in the absence of these groups. Both the density functional theory (DFT) thermochemistry calculations and experimental data support the proposed single electron transfer mechanism with the formation of an acyl radical intermediate in the cross-dehydrogenative coupling. The n-Bu4NI/K2S2O8 mediated oxidative cyclization between 2-aminobenzamide and aldehydes is also reported, with four quinazolin-4(3H)-ones prepared in 65-99% yields.
Keywords: C–N coupling; cross-dehydrogenative coupling; imides; potassium persulfate; quinazolin-4(3H)-ones.
Figures
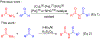
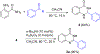
References
-
- Yan S; Appleby T, Larson G; Wu JZ, Hamatake RK, Hong Z, Yao N, Thiazolone-acylsulfonamides as novel HCV NS5B polymerase allosteric inhibitors: Convergence of structure-based drug design and Xray crystallographic study. Bioorg. Med. Chem. Lett 2007, 17, 1991–1995. - PubMed
- Pinto IL, Boyd HF, Hickey DMB, Natural Product Derived Inhibitors of Lipoprotein Associated Phospholipase A2, Synthesis and Activity of Analogues of SB-253514. Bioorg. Med. Chem. Lett 2000, 10, 2015–2017. - PubMed
- Ding G, Jiang L, Guo L, Chen X, Zhang H, Che Y, Pestalazines and Pestalamides, Bioactive Metabolites from the Plant Pathogenic Fungus Pestalotiopsis theae. J. Nat. Prod 2008, 71, 1861–1865. - PubMed
- Lavrard H, Rodriguez F, Delfourne E, Design of granulatimide and isogranulatimide analogues as potential Chk1 inhibitors: Study of amino-platforms for their synthesis. Bioorg. Med. Chem 2014, 22, 4961. - PubMed
-
-
For examples, see:
- Evans DA, Nagorny P, Xu R-S, Ceric Ammonium Nitrate Promoted Oxidation of Oxazoles. Org. Lett 2006, 8, 5669–5671. - PubMed
- Nicolaou KC, Mathison CJN, Synthesis of imides, Nacyl vinylogous carbamates and ureas, and nitriles by oxidation of amides and amines with Dess-Martin periodinane. Angew. Chem., Int. Ed 2005, 44, 5992–5997. - PubMed
- Xu L, Zhang S-H, Trudell ML, Novel chromium(VI)-catalyzed oxidation of N-alkylamides to imides with periodic acid. ChemComm 2004, 14, 1668–1669. - PubMed
- Yu H, Chen Y-G, Zhang Y-H, TBHP/TEMPO-Mediated Oxidative Synthesis of Imides from Amides. Chin. J. Chem 2015, 33, 531–534.
- Chang H-H, X He X, Zang Z-L, Zhou C-H, Cai G-X, Visible-light-driven α-Oxidation of Amide C(sp3)-H Bonds to Imides via N-Bromosuccinimide and Water. Asian J. Org. Chem 2022, 11, e202200500.
- Mei C, Hu Y, Lu W, Visible-Light-Driven Oxidation of N-Alkylamides to Imides Using Oxone/H2O and Catalytic KBr. Synthesis 2018, 50, 2999–3005.
- Wang F, Liu H, Fu H, Jiang Y, Zhao Y, Highly efficient iron(II) chloride/Nbromosuccinimide-mediated synthesis of imides and acylsulfonamides. Adv. Synth. Catal 2009, 351, 246–252.
-
-
- Lorion MM, Duarte FJS, Calhorda MJ, Oble J; Poli G, Opening the Way to Catalytic Aminopalladation/Proxicyclic Dehydropalladation: Access to Methylidene γ-Lactams. Org. Lett 2016, 18, 1020–1023. - PubMed
- Wang J-J, Yu W, Anti-Markovnikov Hydroazidation of Alkenes by Visible-Light Photoredox Catalysis. Chem. Eur. J 2019, 25, 3510–3514 - PubMed
-
- Rajan IAPS, Rajendran S, Amidic resonance not a barrier for transamidation of N-pivaloyl activated amides: catalyst, base and additive free conditions. Org. Biomol. Chem 2023, 21, 4760–4765. - PubMed
- Sivaraj C, Gandhi T, Solvent-controlled amidation of acid chlorides at room temperature: new route to access aromatic primary amides and imides amenable for late-stage functionalization. RSC Adv. 2023, 13, 9231–9236. - PMC - PubMed
-
- Dai J, Day CS, Noftle RE, Synthesis and structural characterization of 3-thienyl alkyl imides. Tetrahedron 2003, 59, 9389–9397.
- Lee J, Hong M, Jung Y, Cho EJ, Rhee H, Synthesis of 1,3,5-trisubstituted-1,2,4-triazoles by microwave-assisted N-acylation of amide derivatives and the consecutive reaction with hydrazine hydrochlorides. Tetrahedron 2012, 68, 2045–2051.
Grants and funding
LinkOut - more resources
Full Text Sources
