Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus
- PMID: 39051200
- PMCID: PMC11270426
- DOI: 10.3390/jdb12030018
Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus
Abstract
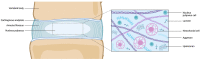
The intervertebral disc (IVD) is the largest avascular organ of the human body and plays a fundamental role in providing the spine with its unique structural and biomechanical functions. The inner part of the IVD contains the nucleus pulposus (NP), a gel-like tissue characterized by a high content of type II collagen and proteoglycans, which is crucial for the disc's load-bearing and shock-absorbing properties. With aging and IVD degeneration (IDD), the NP gradually loses its physiological characteristics, leading to low back pain and additional sequelae. In contrast to surrounding spinal tissues, the NP presents a distinctive embryonic development since it directly derives from the notochord. This review aims to explore the embryology of the NP, emphasizing the pivotal roles of key transcription factors, which guide the differentiation and maintenance of the NP cellular components from the notochord and surrounding sclerotome. Through an understanding of NP development, we sought to investigate the implications of the critical developmental aspects in IVD-related pathologies, such as IDD and the rare malignant chordomas. Moreover, this review discusses the therapeutic strategies targeting these pathways, including the novel regenerative approaches leveraging insights from NP development and embryology to potentially guide future treatments.
Keywords: degeneration; development; embryology; intervertebral disc; notochordal cells; nucleus pulposus; regenerative medicine; transcription factors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Tilotta V., Vadalà G., Ambrosio L., Cicione C., Di Giacomo G., Russo F., Papalia R., Denaro V. Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro. Front. Bioeng. Biotechnol. 2023;11:1152207. doi: 10.3389/fbioe.2023.1152207. - DOI - PMC - PubMed
-
- Risbud M.V., Schoepflin Z.R., Mwale F., Kandel R.A., Grad S., Iatridis J.C., Sakai D., Hoyland J.A. Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J. Orthop. Res. 2015;33:283–293. doi: 10.1002/jor.22789. - DOI - PMC - PubMed
-
- Soma H., Sakai D., Nakamura Y., Tamagawa S., Warita T., Schol J., Matsushita E., Naiki M., Sato M., Watanabe M. Recombinant Laminin-511 Fragment (iMatrix-511) Coating Supports Maintenance of Human Nucleus Pulposus Progenitor Cells In Vitro. Int. J. Mol. Sci. 2023;24:16713. doi: 10.3390/ijms242316713. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

