177Lu Anti-Angiogenic Radioimmunotherapy Targeting ATP Synthase in Gastric Cancer Model
- PMID: 39051327
- PMCID: PMC11270205
- DOI: 10.3390/antib13030051
177Lu Anti-Angiogenic Radioimmunotherapy Targeting ATP Synthase in Gastric Cancer Model
Abstract
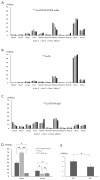
This study investigated a novel radioimmunotherapy strategy for targeting tumor angiogenesis. We developed a radiopharmaceutical complex by labeling an anti-adenosine triphosphate synthase (ATPS) monoclonal antibody (mAb) with the radioisotope 177Lu using DOTA as a chelating agent. 177Lu-DOTA-ATPS mAb demonstrated high labeling efficiency (99.0%) and stability in serum. MKN-45 cancer cells exhibited the highest cellular uptake, which could be specifically blocked by unlabeled ATPS mAb. In mice, 177Lu-DOTA-ATPS mAb accumulated significantly in tumors, with a tumor uptake of 16.0 ± 1.5%ID/g on day 7. 177Lu-DOTA-ATPS mAb treatment significantly reduced the viability of MKN-45 cells in a dose-dependent manner. In a xenograft tumor model, this radioimmunotherapy strategy led to substantial tumor growth inhibition (82.8%). Furthermore, combining 177Lu-DOTA-ATPS mAb with sunitinib, an anti-angiogenic drug, enhanced the therapeutic efficacy of sunitinib in the mouse model. Our study successfully developed 177Lu-DOTA-ATPS mAb, a radioimmunotherapy agent targeting tumor blood vessels. This approach demonstrates significant promise for inhibiting tumor growth, both as a single therapy and in combination with other anti-cancer drugs.
Keywords: 177Lu; ATP synthase; angiogenesis; gastric cancer; radioimmunotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

