Rapid depletion of "catch-and-release" anti-ASGR1 antibody in vivo
- PMID: 39051531
- PMCID: PMC11275528
- DOI: 10.1080/19420862.2024.2383013
Rapid depletion of "catch-and-release" anti-ASGR1 antibody in vivo
Abstract
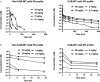
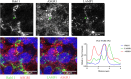
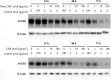
Targeting antigens with antibodies exhibiting pH/Ca2+-dependent binding against an antigen is an attractive strategy to mitigate target-mediated disposition and antigen buffering. Studies have reported improved serum exposure of antibodies exhibiting pH/Ca2+-binding against membrane-bound receptors. Asialoglycoprotein receptor 1 (ASGR1) is a membrane-bound receptor primarily localized in hepatocytes. With a high expression level of approximately one million receptors per cell, high turnover, and rapid recycling, targeting this receptor with a conventional antibody is a challenge. In this study, we identified an antibody exhibiting pH/Ca2+-dependent binding to ASGR1 and generated antibody variants with increased binding to neonatal crystallizable fragment receptor (FcRn). Serum exposures of the generated anti-ASGR1 antibodies were analyzed in transgenic mice expressing human FcRn. Contrary to published reports of increased serum exposure of pH/Ca2+-dependent antibodies, the pH/Ca2+-dependent anti-ASGR1 antibody had rapid serum clearance in comparison to a conventional anti-ASGR1 antibody. We conducted sub-cellular trafficking studies of the anti-ASGR1 antibodies along with receptor quantification analysis for mechanistic understanding of the rapid serum clearance of pH/Ca2+-dependent anti-ASGR1 antibody. The findings from our study provide valuable insights in identifying the antigens, especially membrane bound, that may benefit from targeting with pH/Ca2+-dependent antibodies to obtain increased serum exposure.
Keywords: Antigen-antibody trafficking; TMDD; fluorescence microscopy; pH/Ca2+-dependent antibodies; pharmacokinetics.
Conflict of interest statement
The authors declare the following competing financial interest: All authors, except M.D are full time employees and shareholders of Amgen Inc. M.D is a shareholder of Amgen Inc.
Figures
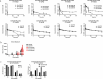


Similar articles
-
Maximizing in vivo target clearance by design of pH-dependent target binding antibodies with altered affinity to FcRn.MAbs. 2017 Oct;9(7):1105-1117. doi: 10.1080/19420862.2017.1359455. Epub 2017 Aug 8. MAbs. 2017. PMID: 28786732 Free PMC article.
-
A Minimal Physiologically Based Pharmacokinetic Model with a Nested Endosome Compartment for Novel Engineered Antibodies.AAPS J. 2018 Mar 14;20(3):48. doi: 10.1208/s12248-017-0183-4. AAPS J. 2018. PMID: 29541870 Free PMC article.
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
A novel approach to investigate the effect of methionine oxidation on pharmacokinetic properties of therapeutic antibodies.MAbs. 2014;6(5):1229-42. doi: 10.4161/mabs.29601. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517308 Free PMC article.
-
Targeting FcRn for the modulation of antibody dynamics.Mol Immunol. 2015 Oct;67(2 Pt A):131-41. doi: 10.1016/j.molimm.2015.02.007. Epub 2015 Mar 9. Mol Immunol. 2015. PMID: 25766596 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
